Bifidobacterium lactis attenuates onset of inflammation in a murine model of colitis
- PMID: 21274375
- PMCID: PMC3027012
- DOI: 10.3748/wjg.v17.i4.459
Bifidobacterium lactis attenuates onset of inflammation in a murine model of colitis
Abstract
Aim: To assess the anti-inflammatory effect of the probiotic Bifidobacterium lactis (B. lactis) in an adoptive transfer model of colitis.
Methods: Donor and recipient mice received either B. lactis or bacterial culture medium as control (deMan Rogosa Sharpe) in drinking water for one week prior to transfer of a mix of naive and regulatory T cells until sacrifice.
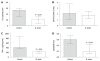
Results: All recipient mice developed signs of colonic inflammation, but a significant reduction of weight loss was observed in B. lactis-fed recipient mice compared to control mice. Moreover, a trend toward a diminution of mucosal thickness and attenuated epithelial damage was revealed. Colonic expression of pro-inflammatory and T cell markers was significantly reduced in B. lactis-fed recipient mice compared to controls. Concomitantly, forkhead box protein 3, a marker of regulatory T cells, was significantly up-regulated by B. lactis.
Conclusion: Daily oral administration of B. lactis was able to reduce inflammatory and T cells mediators and to promote regulatory T cells specific markers in a mouse model of colitis.
Keywords: Adoptive transfer model; Bifidobacterium; Colitis; Inflammation; Mice; Probiotics; Regulatory T cells.
Figures





Similar articles
-
Treatment with Bifidobacterium bifidum 17 partially protects mice from Th1-driven inflammation in a chemically induced model of colitis.Int J Food Microbiol. 2011 Sep 1;149(1):45-9. doi: 10.1016/j.ijfoodmicro.2010.12.020. Epub 2010 Dec 31. Int J Food Microbiol. 2011. PMID: 21257218
-
Bifidobacterium infantis attenuates colitis by regulating T cell subset responses.World J Gastroenterol. 2014 Dec 28;20(48):18316-29. doi: 10.3748/wjg.v20.i48.18316. World J Gastroenterol. 2014. PMID: 25561798 Free PMC article.
-
Probiotic administration alters the gut flora and attenuates colitis in mice administered dextran sodium sulfate.J Gastroenterol Hepatol. 2008 Dec;23(12):1834-9. doi: 10.1111/j.1440-1746.2008.05723.x. J Gastroenterol Hepatol. 2008. PMID: 19120873
-
A comparative study of the preventative effects exerted by three probiotics, Bifidobacterium lactis, Lactobacillus casei and Lactobacillus acidophilus, in the TNBS model of rat colitis.J Appl Microbiol. 2007 Oct;103(4):836-44. doi: 10.1111/j.1365-2672.2007.03302.x. J Appl Microbiol. 2007. PMID: 17897185
-
Probiotic Bifidobacterium breve induces IL-10-producing Tr1 cells in the colon.PLoS Pathog. 2012;8(5):e1002714. doi: 10.1371/journal.ppat.1002714. Epub 2012 May 31. PLoS Pathog. 2012. PMID: 22693446 Free PMC article.
Cited by
-
Nicotinamide Ameliorates Dextran Sulfate Sodium-Induced Chronic Colitis in Mice through Its Anti-Inflammatory Properties and Modulates the Gut Microbiota.J Immunol Res. 2021 Mar 6;2021:5084713. doi: 10.1155/2021/5084713. eCollection 2021. J Immunol Res. 2021. PMID: 33748287 Free PMC article.
-
Host lysozyme-mediated lysis of Lactococcus lactis facilitates delivery of colitis-attenuating superoxide dismutase to inflamed colons.Proc Natl Acad Sci U S A. 2015 Jun 23;112(25):7803-8. doi: 10.1073/pnas.1501897112. Epub 2015 Jun 8. Proc Natl Acad Sci U S A. 2015. PMID: 26056274 Free PMC article.
-
Intestinal virome changes precede autoimmunity in type I diabetes-susceptible children.Proc Natl Acad Sci U S A. 2017 Jul 25;114(30):E6166-E6175. doi: 10.1073/pnas.1706359114. Epub 2017 Jul 10. Proc Natl Acad Sci U S A. 2017. PMID: 28696303 Free PMC article.
-
Effect of Crohn's disease mesenteric mesenchymal stem cells and their extracellular vesicles on T-cell immunosuppressive capacity.J Cell Mol Med. 2022 Oct;26(19):4924-4939. doi: 10.1111/jcmm.17483. Epub 2022 Sep 1. J Cell Mol Med. 2022. PMID: 36047483 Free PMC article.
-
Synbiotic Effect of Bifidobacterium lactis CNCM I-3446 and Bovine Milk-Derived Oligosaccharides on Infant Gut Microbiota.Nutrients. 2020 Jul 29;12(8):2268. doi: 10.3390/nu12082268. Nutrients. 2020. PMID: 32751149 Free PMC article.
References
-
- Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol. 2003;3:521–533. - PubMed
-
- Yu QT, Saruta M, Avanesyan A, Fleshner PR, Banham AH, Papadakis KA. Expression and functional characterization of FOXP3+ CD4+ regulatory T cells in ulcerative colitis. Inflamm Bowel Dis. 2007;13:191–199. - PubMed
-
- MacDonald TT. Effector and regulatory lymphoid cells and cytokines in mucosal sites. Curr Top Microbiol Immunol. 1999;236:113–135. - PubMed
-
- Blumberg RS, Saubermann LJ, Strober W. Animal models of mucosal inflammation and their relation to human inflammatory bowel disease. Curr Opin Immunol. 1999;11:648–656. - PubMed
-
- Linskens RK, Huijsdens XW, Savelkoul PH, Vandenbroucke-Grauls CM, Meuwissen SG. The bacterial flora in inflammatory bowel disease: current insights in pathogenesis and the influence of antibiotics and probiotics. Scand J Gastroenterol Suppl. 2001:29–40. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

