Loss of the NHE2 Na+/H+ exchanger in mice results in dilation of folliculo-stellate cell canaliculi
- PMID: 21274460
- PMCID: PMC3025390
- DOI: 10.1155/2011/510827
Loss of the NHE2 Na+/H+ exchanger in mice results in dilation of folliculo-stellate cell canaliculi
Abstract
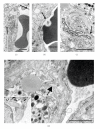
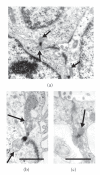
Genetic ablation of the NHE2 Na+/H+ exchanger causes gastric achlorhydria, absorptive defects in kidney and colon, and low fertility. Here we show that NHE2 is expressed in the pituitary, with the highest mRNA expression in pars distalis and lower expression in pars intermedia. In pars distalis of NHE2-null mice, prominent cyst-like dilatations of folliculo-stellate (FS) cell canaliculi developed with age, and there were increased FS cell area, accumulation of lipid in FS cell cytoplasm, redundancies in FS cell basement membrane, and other changes. The expansion of the canaliculi indicates that NHE2 is a major absorptive Na+/H+ exchanger in the luminal membranes lining the extensive network of channels formed by FS cells, which may provide a means of intrapituitary communication. The results suggest that NHE2 contributes to homeostatic regulation of the volume and composition of the canalicular fluid and may counter the secretory activity of the CFTR Cl⁻ channel, which is known to be expressed in pituitary.
Figures







References
-
- Herbert DC, Silverman AY. Topographical distribution of the gonadotrophs, mammotrophs, somatotrophs and thyrotrophs in the pituitary gland of the baboon (Papio cynocephalus) Cell and Tissue Research. 1983;230(1):233–238. - PubMed
-
- Allaerts W, Carmeliet P, Denef C. New perspectives in the function of pituitary folliculo-stellate cells. Molecular and Cellular Endocrinology. 1990;71(2):73–81. - PubMed
-
- Inoue K, Couch EF, Takano K, Ogawa S. The structure and function of folliculo-stellate cells in the anterior pituitary gland. Archives of Histology and Cytology. 1999;62(3):205–218. - PubMed
-
- Ferrara N, Fujii DK, Goldsmith PC. Transport epithelial characteristics of cultured bovine pituitary follicular cells. American Journal of Physiology. 1987;252(3, part 1):E304–E312. - PubMed
-
- Ferrara N, Gospodarowicz D. Regulation of ion transport in hypophysial pars intermedia follicular cell monolayers. Biochemical and Biophysical Research Communications. 1988;157(3):1376–1382. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

