Chromatin configuration and epigenetic landscape at the sex chromosome bivalent during equine spermatogenesis
- PMID: 21274552
- PMCID: PMC3100478
- DOI: 10.1007/s00412-010-0306-5
Chromatin configuration and epigenetic landscape at the sex chromosome bivalent during equine spermatogenesis
Abstract
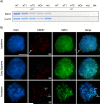
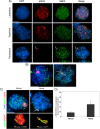
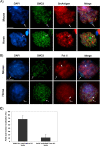
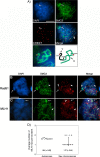
Pairing of the sex chromosomes during mammalian meiosis is characterized by the formation of a unique heterochromatin structure at the XY body. The mechanisms underlying the formation of this nuclear domain are reportedly highly conserved from marsupials to mammals. In this study, we demonstrate that in contrast to all eutherian species studied to date, partial synapsis of the heterologous sex chromosomes during pachytene stage in the horse is not associated with the formation of a typical macrochromatin domain at the XY body. While phosphorylated histone H2AX (γH2AX) and macroH2A1.2 are present as a diffuse signal over the entire macrochromatin domain in mouse pachytene spermatocytes, γH2AX, macroH2A1.2, and the cohesin subunit SMC3 are preferentially enriched at meiotic sex chromosome cores in equine spermatocytes. Moreover, although several histone modifications associated with this nuclear domain in the mouse such as H3K4me2 and ubH2A are conspicuously absent in the equine XY body, prominent RNA polymerase II foci persist at the sex chromosomes. Thus, the localization of key marker proteins and histone modifications associated with the XY body in the horse differs significantly from all other mammalian systems described. These results demonstrate that the epigenetic landscape and heterochromatinization of the equine XY body might be regulated by alternative mechanisms and that some features of XY body formation may be evolutionary divergent in the domestic horse. We propose equine spermatogenesis as a unique model system for the study of the regulatory networks leading to the epigenetic control of gene expression during XY body formation.
Figures




References
-
- Akhmedov A, Gross B, Jessberger R. Mammalian smc3 c-terminal and coiled-coil protein domains specifically bind palindromic DNA, do not block DNA ends, and prevent DNA bending. J Biol Chem. 1999;274(53):38216–38224. - PubMed
-
- Anderson SF, Schlegel BP, Nakajima T, Wolpin ES, Parvin JD. BRCA1 protein is linked to the RNA polymerase Π holoenzyme complex via RNA helicase A. Nat Genet. 1998;19:254–256. - PubMed
-
- Baarends W, Hoogerbrugge J, Roest H, Ooms M, Vreeburg J, Hoeijmakers J, Grootegoed J. Histone ubiquitination and chromatin remodeling in mouse spermatogenesis. Dev Biol. 1999;207(2):322–333. - PubMed
-
- Bannister L, Schimenti J. Homologous recombinational repair proteins in mouse meiosis. Cytogenet Genome Res. 2004;107(3–4):191–200. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

