Imaging for the diagnosis and management of traumatic brain injury
- PMID: 21274684
- PMCID: PMC3026928
- DOI: 10.1007/s13311-010-0003-3
Imaging for the diagnosis and management of traumatic brain injury
Abstract
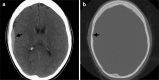
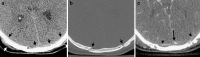
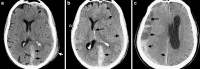
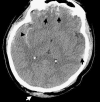
To understand the role of imaging in traumatic brain injury (TBI), it is important to appreciate that TBI encompasses a heterogeneous group of intracranial injuries and includes both insults at the time of impact and a deleterious secondary cascade of insults that require optimal medical and surgical management. Initial imaging identifies the acute primary insult that is essential to diagnosing TBI, but serial imaging surveillance is also critical to identifying secondary injuries such as cerebral herniation and swelling that guide neurocritical management. Computed tomography (CT) is the mainstay of TBI imaging in the acute setting, but magnetic resonance tomography (MRI) has better diagnostic sensitivity for nonhemorrhagic contusions and shear-strain injuries. Both CT and MRI can be used to prognosticate clinical outcome, and there is particular interest in advanced applications of both techniques that may greatly improve the sensitivity of conventional CT and MRI for both the diagnosis and prognosis of TBI.
Figures


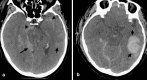





References
-
- Centers for Disease Control and Prevention. Traumatic brain injury. Available at: http://wwwcdcgov/traumaticbraininjury/statisticshtml Last modified March 17, 2010.
-
- Bratton SL, Chestnut RM, Ghajar J, et al. Guidelines for the management of severe traumatic brain injury. IX. Cerebral perfusion thresholds. J Neurotrauma. 2007;24(1):S59–S64. - PubMed

