Activation of alpha(1) -adrenergic receptors stimulate the growth of small mouse cholangiocytes via calcium-dependent activation of nuclear factor of activated T cells 2 and specificity protein 1
- PMID: 21274883
- PMCID: PMC3522188
- DOI: 10.1002/hep.24041
Activation of alpha(1) -adrenergic receptors stimulate the growth of small mouse cholangiocytes via calcium-dependent activation of nuclear factor of activated T cells 2 and specificity protein 1
Abstract
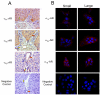
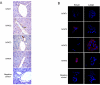
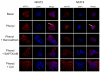
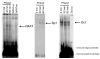
Small cholangiocytes proliferate via activation of calcium (Ca(2+) )-dependent signaling in response to pathological conditions that trigger the damage of large cyclic adenosine monophosphate-dependent cholangiocytes. Although our previous studies suggest that small cholangiocyte proliferation is regulated by the activation of Ca(2+) -dependent signaling, the intracellular mechanisms regulating small cholangiocyte proliferation are undefined. Therefore, we sought to address the role and mechanisms of action by which phenylephrine, an α(1) -adrenergic agonist stimulating intracellular D-myo-inositol-1,4,5-triphosphate (IP(3) )/Ca(2+) levels, regulates small cholangiocyte proliferation. Small and large bile ducts and cholangiocytes expressed all AR receptor subtypes. Small (but not large) cholangiocytes respond to phenylephrine with increased proliferation via the activation of IP(3) /Ca(2+) -dependent signaling. Phenylephrine stimulated the production of intracellular IP(3) . The Ca(2+) -dependent transcription factors, nuclear factor of activated T cells 2 (NFAT2) and NFAT4, were predominantly expressed by small bile ducts and small cholangiocytes. Phenylephrine stimulated the Ca(2+) -dependent DNA-binding activities of NFAT2, NFAT4, and Sp1 (but not Sp3) and the nuclear translocation of NFAT2 and NFAT4 in small cholangiocytes. To determine the relative roles of NFAT2, NFAT4, or Sp1, we knocked down the expression of these transcription factors with small hairpin RNA. We observed an inhibition of phenylephrine-induced proliferation in small cholangiocytes lacking the expression of NFAT2 or Sp1. Phenylephrine stimulated small cholangiocyte proliferation is regulated by Ca(2+) -dependent activation of NFAT2 and Sp1.
Conclusion: Selective stimulation of Ca(2+) -dependent small cholangiocyte proliferation may be key to promote the repopulation of the biliary epithelium when large bile ducts are damaged during cholestasis or by toxins.
Copyright © 2010 American Association for the Study of Liver Diseases.
Figures





References
-
- Alpini G, Prall RT, LaRusso NF. The pathobiology of biliary epithelia. In: Arias IM, Boyer JL, Chisari FV, Fausto N, Jakoby W, Schachter D, Shafritz DA, editors. The Liver; Biology & Pathobiology. 4th Ed Lippincott Williams & Wilkins; Philadelphia, PA: 2001. pp. 421–435.
-
- Alpini G, Glaser S, Ueno Y, Pham L, Podila PV, Caligiuri A, LeSage G, et al. Heterogeneity of the proliferative capacity of rat cholangiocytes after bile duct ligation. Am J Physiol Gastrointest Liver Physiol. 1998;274:G767–775. - PubMed
-
- LeSage G, Glaser S, Marucci L, Benedetti A, Phinizy JL, Rodgers R, Caligiuri A, et al. Acute carbon tetrachloride feeding induces damage of large but not small cholangiocytes from BDL rat liver. Am J Physiol Gastrointest Liver Physiol. 1999;276:G1289–1301. - PubMed
-
- Francis H, Glaser S, Ueno Y, LeSage G, Marucci L, Benedetti A, Taffetani S, et al. cAMP stimulates the secretory and proliferative capacity of the rat intrahepatic biliary epithelium through changes in the PKA/Src/MEK/ERK1/2 pathway. J Hepatol. 2004;41:528–537. - PubMed
-
- Ueno Y, Alpini G, Yahagi K, Kanno N, Moritoki Y, Fukushima K, Glaser S, et al. Evaluation of differential gene expression by microarray analysis in small and large cholangiocytes isolated from normal mice. Liver Int. 2003;23:449–459. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
