Visualization of protein interactions in living Drosophila embryos by the bimolecular fluorescence complementation assay
- PMID: 21276241
- PMCID: PMC3041725
- DOI: 10.1186/1741-7007-9-5
Visualization of protein interactions in living Drosophila embryos by the bimolecular fluorescence complementation assay
Abstract
Background: Protein interactions control the regulatory networks underlying developmental processes. The understanding of developmental complexity will, therefore, require the characterization of protein interactions within their proper environment. The bimolecular fluorescence complementation (BiFC) technology offers this possibility as it enables the direct visualization of protein interactions in living cells. However, its potential has rarely been applied in embryos of animal model organisms and was only performed under transient protein expression levels.
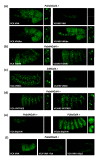
Results: Using a Hox protein partnership as a test case, we investigated the suitability of BiFC for the study of protein interactions in the living Drosophila embryo. Importantly, all BiFC parameters were established with constructs that were stably expressed under the control of endogenous promoters. Under these physiological conditions, we showed that BiFC is specific and sensitive enough to analyse dynamic protein interactions. We next used BiFC in a candidate interaction screen, which led to the identification of several Hox protein partners.
Conclusion: Our results establish the general suitability of BiFC for revealing and studying protein interactions in their physiological context during the rapid course of Drosophila embryonic development.
Figures








References
-
- Prasher DC, Eckenrode VK, Ward WW, Prendergast FG, Cormier MJ. Primary structure of the Aequorea victoria green-fluorescent protein. Gene. 1992;111(2):229–233. - PubMed
-
- Ghosh Indraneel, Hamilton AD, Lynn Regan. Antiparallel Leucine Zipper-Directed Protein Reassembly: Application to the Green Fluorescent Protein. J Am Chem Soc. 2000;122:5658–5659.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

