The vitamin D receptor: a tumor suppressor in skin
- PMID: 21276406
- PMCID: PMC4113511
The vitamin D receptor: a tumor suppressor in skin
Abstract
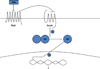
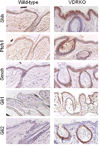
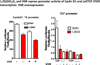
Epidemiologic evidence supporting a major chemopreventive role for vitamin D in various malignancies is strong. Likewise the use of the active metabolite of vitamin D, 1,25(OH)(2)D(3), and its analogs to prevent and/or treat a wide variety of malignancies in animals is well established. The evidence has been less compelling for epidermal carcinogenesis perhaps because the same agent that produces vitamin D in the skin, UVB radiation (UVR), is also the same agent that results in most epidermal malignancies. However, recent studies indicate that the role of vitamin D and its receptor (VDR) in protecting against the development of epidermal tumors deserves a closer look. One such study found mice lacking the VDR were quite sensitive to epidermal tumor formation following the administration of the carcinogen DMBA. A more recent study showed that these mice were similarly more sensitive to tumor formation following UVR, results we have confirmed. The epidermis of the VDR null mouse is hyperproliferative with gross distortion of hair follicles, structures that may provide the origin for the tumors found in the skin following such treatment. Two interacting pathways critical for epidermal and hair follicle function, beta-catenin and hedgehog (Hh), result in epidermal tumors when they are activated abnormally. Thus, we considered the possibility that loss of VDR predisposes to epidermal tumor formation by activation of either or both beta-catenin and Hh signaling. We determined that all elements of the Hh signaling pathway are upregulated in the epidermis and utricles of the VDR null mouse, and that 1,25(OH)(2)D(3) suppresses the expression of these elements in normal mouse skin. In addition we observed that the transcriptional activity of beta-catenin was increased in keratinocytes lacking the VDR. These results lead us to the hypothesis that the VDR with its ligand 1,25(OH)(2)D(3) functions as a tumor suppressor with respect to epidermal tumor formation in response to UVR by regulating Hh and beta-catenin signaling.
Conflict of interest statement
The authors report no conflicts of interest.
Figures






References
-
- Aszterbaum M, Epstein J, Oro A, Douglas V, Leboit PE, Scott MP, Epstein EH., Jr Ultraviolet and ionizing radiation enhance the growth of BCCs and trichoblastomas in patched heterozygous knockout mice. Nat Med. 1999;5(11):1285–1291. - PubMed
-
- Aszterbaum M, Rothman A, Johnson RL, Fisher M, Xie J, Bonifas JM, Zhang X, Scott MP, Epstein EH., Jr Identification of mutations in the human PATCHED gene in sporadic basal cell carcinomas and in patients with the basal cell nevus syndrome. J Invest Dermatol. 1998;110(6):885–888. - PubMed
-
- Barnfield PC, Zhang X, Thanabalasingham V, Yoshida M, Hui CC. Negative regulation of Gli1 and Gli2 activator function by Suppressor of fused through multiple mechanisms. Differentiation. 2005;73(8):397–405. - PubMed
-
- Bienz M. beta-Catenin: a pivot between cell adhesion and Wnt signalling. Curr Biol. 2005;15(2):R64–R67. - PubMed

