A phase II, single-arm study of the anti-α5β1 integrin antibody volociximab as monotherapy in patients with platinum-resistant advanced epithelial ovarian or primary peritoneal cancer
- PMID: 21276608
- PMCID: PMC4426879
- DOI: 10.1016/j.ygyno.2010.12.362
A phase II, single-arm study of the anti-α5β1 integrin antibody volociximab as monotherapy in patients with platinum-resistant advanced epithelial ovarian or primary peritoneal cancer
Abstract
Objective: This phase II, multicenter, single-arm, two-stage study in platinum-resistant, advanced epithelial ovarian or primary peritoneal cancer evaluated the efficacy, safety, and tolerability of weekly single-agent volociximab. Pharmacokinetic/pharmacodynamic (PK/PD) studies were also performed.
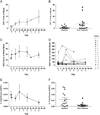
Methods: Sixteen patients were enrolled in Stage 1. Volociximab was administered at 15mg/kg IV qwk until progression of disease or drug intolerability. Tumor response was assessed every 8weeks. Serum samples for PK or whole blood for the evaluation of circulating tumor cells, endothelial cells, and endothelial progenitor cells were obtained on Days 1, 8, 15, 29, and 50. Ascites from one patient was collected for volociximab concentration analysis. Archived tumor tissue was analyzed by immunohistochemistry (IHC) for α5 integrin expression.
Results: Safety data are available on all 16 patients; 14 were evaluable for efficacy. One patient had stable disease at 8weeks. The remaining 13 progressed on treatment. Twelve patients (75%) experienced study-related adverse events (AEs); the most common (≥20%) were headache and fatigue. Three patients experienced possible study-related serious AEs (SAEs): reversible posterior leukoencephalopathy syndrome, pulmonary embolism, and hyponatremia. Peak serum concentrations of volociximab increased 2-3 fold from Day 1 to Day 50. Clinically relevant trough levels were achieved (>150μg/mL). IHC analysis of archived tumor sections showed low-to-moderate expression of α5 integrin on all ovarian cancer tissue evaluated.
Conclusion: Despite insufficient clinical activity in this refractory patient population to continue the study, weekly volociximab was well tolerated, and the gained understanding of the mechanism of action of volociximab will inform future development efforts.
Copyright © 2010 Elsevier Inc. All rights reserved.
Conflict of interest statement
Katherine M. Bell-McGuinn: None.
Carolyn M. Matthews: None.
Minal Barve: None.
Lucy Gilbert: None.
Russell J. Schilder: None.
Figures


References
-
- Francis SE, Goh KL, Hodivala-Dilke K, et al. Central roles of alpha5beta1 integrin and fibronectin in vascular development in mouse embryos and embryoid bodies. Arterioscler Thromb Vasc Biol. 2002;22:927–933. - PubMed
-
- Ramakrishnan V, Bhaskar V, Law DA, et al. Preclinical evaluation of an anti-alpha5beta1 integrin antibody as a novel anti-angiogenic agent. J Exp Ther Oncol. 2006;5:273–286. - PubMed
-
- Markowska J, Szala S. Inhibitors of angiogenesis in therapy of ovarian cancers. Eur J Gynaecol Oncol. 2004;25:562–567. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

