NADH fluorescence lifetime analysis of the effect of magnesium ions on ALDH2
- PMID: 21276780
- PMCID: PMC3103607
- DOI: 10.1016/j.cbi.2011.01.023
NADH fluorescence lifetime analysis of the effect of magnesium ions on ALDH2
Abstract
Aldehyde dehydrogenase 2 (ALDH2) catalyzes oxidation of toxic aldehydes to carboxylic acids. Physiologic levels of Mg(2+) ions influence ALDH2 activity in part by increasing NADH binding affinity. Traditional fluorescence measurements monitor the blue shift of the NADH fluorescence spectrum to study ALDH2-NADH interactions. By using time-resolved fluorescence spectroscopy, we have resolved the fluorescent lifetimes (τ) of free NADH (τ=0.4 ns) and bound NADH (τ=6.0 ns). We used this technique to investigate the effects of Mg(2+) on the ALDH2-NADH binding characteristics and enzyme catalysis. From the resolved free and bound NADH fluorescence signatures, the K(D) for NADH with ALDH2 ranged from 468 μM to 12 μM for Mg(2+) ion concentrations of 20 to 6000 μM, respectively. The rate constant for dissociation of the enzyme-NADH complex ranged from 0.4s(-1) (6000 μM Mg(2+)) to 8.3s(-1) (0 μM Mg(2+)) as determined by addition of excess NAD(+) to prevent re-association of NADH and resolving the real-time NADH fluorescence signal. The apparent NADH association/re-association rate constants were approximately 0.04 μM(-1)s(-1) over the entire Mg(2+) ion concentration range and demonstrate that Mg(2+) ions slow the release of NADH from the enzyme rather than promoting its re-association. We applied NADH fluorescence lifetime analysis to the study of NADH binding during enzyme catalysis. Our fluorescence lifetime analysis confirmed complex behavior of the enzyme activity as a function of Mg(2+) concentration. Importantly, we observed no pre-steady state burst of NADH formation. Furthermore, we observed distinct fluorescence signatures from multiple ALDH2-NADH complexes corresponding to free NADH, enzyme-bound NADH, and, potentially, an abortive NADH-enzyme-propanal complex (τ=11.2 ns).
Copyright © 2011 Elsevier Ireland Ltd. All rights reserved.
Figures







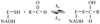
References
-
- Ho KK, Alli-Hassani A, Hurley TD, Weiner H. Differential effects of Mg2+ ions on the individual kinetic steps of human cytosolic and mitochondrial aldehyde dehydrogenases. Biochemistry. 2005;44:8022–8029. - PubMed
-
- Brichac J, Ho KK, Honzatko A, Wang R, Lu X, Weiner H, Picklo MJ., Sr Enantioselective oxidation of trans-4-hydroxy-2-nonenal is aldehyde dehydrogenase isozyme and Mg2+ dependent. Chem. Res. Toxicol. 2007;20:887–895. - PubMed
-
- Wang SL, Wu CW, Cheng TC, Yin SJ. Isolation of high-Km aldehyde dehydrogenase isoenzymes from human gastric mucosa. Biochem. Int. 1990;22:199–204. - PubMed
-
- Bennett AF, Buckley PD, Blackwell LF. Inhibition of the dehydrogenase activity of sheep liver cytoplasmic aldehyde dehydrogenase by magnesium ions. Biochemistry. 1983;22:776–784. - PubMed
-
- Takahashi K, Weiner H. Magnesium stimulation of catalytic activity of horse liver aldehyde dehydrogenase. Changes in molecular weight and catalytic sites. J. Biol. Chem. 1980;255:8206–8209. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

