A chemical genetics approach reveals H,K-ATPase-mediated membrane voltage is required for planarian head regeneration
- PMID: 21276941
- PMCID: PMC3278711
- DOI: 10.1016/j.chembiol.2010.11.012
A chemical genetics approach reveals H,K-ATPase-mediated membrane voltage is required for planarian head regeneration
Abstract
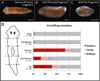
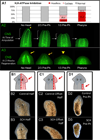
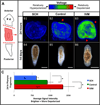
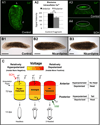
Biophysical signaling is required for both embryonic polarity and regenerative outgrowth. Exploiting endogenous ion transport for regenerative therapies will require direct regulation of membrane voltage. Here, we develop a pharmacological method to target ion transporters, uncovering a role for membrane voltage as a key regulator of anterior polarity in regenerating planaria. Utilizing the highly specific inhibitor, SCH-28080, our data reveal that H(+),K(+)-ATPase-mediated membrane depolarization is essential for anterior gene expression and brain induction. H(+),K(+)-ATPase-independent manipulation of membrane potential with ivermectin confirms that depolarization drives head formation, even at posterior-facing wounds. Using this chemical genetics approach, we demonstrate that membrane voltage controls head-versus-tail identity during planarian regeneration. Our data suggest well-characterized drugs (already approved for human use) might be exploited to control adult stem cell-driven pattern formation during the regeneration of complex structures.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures



Comment in
-
A jump-start for planarian head regeneration.Chem Biol. 2011 Jan 28;18(1):4-6. doi: 10.1016/j.chembiol.2011.01.002. Chem Biol. 2011. PMID: 21276932
References
-
- Adams DS. A new tool for tissue engineers: ions as regulators of morphogenesis during development and regeneration. Tissue Eng Part A. 2008;14:1461–1468. - PubMed
-
- Adams DS, Levin M. Strategies and Techniques for Investigation of Biophysical Signals in Patterning. In: Whitman M, Sater AK, editors. Analysis of Growth Factor Signaling in Embryos. CRC Press; 2006b. pp. 177–262.
-
- Adams DS, Masi A, Levin M. H+ pump-dependent changes in membrane voltage are an early mechanism necessary and sufficient to induce Xenopus tail regeneration. Development. 2007;134:1323–1335. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

