Doxorubicin loaded iron oxide nanoparticles overcome multidrug resistance in cancer in vitro
- PMID: 21277920
- PMCID: PMC3110619
- DOI: 10.1016/j.jconrel.2011.01.024
Doxorubicin loaded iron oxide nanoparticles overcome multidrug resistance in cancer in vitro
Abstract
Multidrug resistance (MDR) is characterized by the overexpression of ATP-binding cassette (ABC) transporters that actively pump a broad class of hydrophobic chemotherapeutic drugs out of cancer cells. MDR is a major mechanism of treatment resistance in a variety of human tumors, and clinically applicable strategies to circumvent MDR remain to be characterized. Here we describe the fabrication and characterization of a drug-loaded iron oxide nanoparticle designed to circumvent MDR. Doxorubicin (DOX), an anthracycline antibiotic commonly used in cancer chemotherapy and substrate for ABC-mediated drug efflux, was covalently bound to polyethylenimine via a pH sensitive hydrazone linkage and conjugated to an iron oxide nanoparticle coated with amine terminated polyethylene glycol. Drug loading, physiochemical properties and pH lability of the DOX-hydrazone linkage were evaluated in vitro. Nanoparticle uptake, retention, and dose-dependent effects on viability were compared in wild-type and DOX-resistant ABC transporter over-expressing rat glioma C6 cells. We found that DOX release from nanoparticles was greatest at acidic pH, indicative of cleavage of the hydrazone linkage. DOX-conjugated nanoparticles were readily taken up by wild-type and drug-resistant cells. In contrast to free drug, DOX-conjugated nanoparticles persisted in drug-resistant cells, indicating that they were not subject to drug efflux. Greater retention of DOX-conjugated nanoparticles was accompanied by reduction of viability relative to cells treated with free drug. Our results suggest that DOX-conjugated nanoparticles could improve the efficacy of chemotherapy by circumventing MDR.
Copyright © 2011 Elsevier B.V. All rights reserved.
Figures




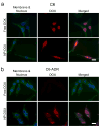
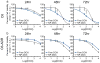

References
-
- Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2002;2(1):48–58. - PubMed
-
- Szakacs G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM. Targeting multidrug resistance in cancer. Nat Rev Drug Discov. 2006;5(3):219–234. - PubMed
-
- Davis ME, Chen Z, Shin DM. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat Rev Drug Discov. 2008;7(9):771–782. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

