Effects of T- and R-state stabilization on deoxyhemoglobin-nitrite reactions and stimulation of nitric oxide signaling
- PMID: 21277987
- PMCID: PMC3115472
- DOI: 10.1016/j.niox.2011.01.006
Effects of T- and R-state stabilization on deoxyhemoglobin-nitrite reactions and stimulation of nitric oxide signaling
Abstract
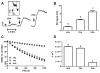
Recent data suggest that transitions between the relaxed (R) and tense (T) state of hemoglobin control the reduction of nitrite to nitric oxide (NO) by deoxyhemoglobin. This reaction may play a role in physiologic NO homeostasis and be a novel consideration for the development of the next generation of hemoglobin-based blood oxygen carriers (HBOCs, i.e. artificial blood substitutes). Herein we tested the effects of chemical stabilization of bovine hemoglobin in either the T- (THb) or R-state (RHb) on nitrite-reduction kinetics, NO-gas formation and ability to stimulate NO-dependent signaling. These studies were performed over a range of fractional saturations that is expected to mimic biological conditions. The initial rate for nitrite-reduction decreased in the following order RHb>bHb>THb, consistent with the hypothesis that the rate constant for nitrite reduction is faster with R-state Hb and slower with T-state Hb. Moreover, RHb produced more NO-gas and inhibited mitochondrial respiration more potently than both bHb and THb. Interestingly, at low oxygen fractional saturations, THb produced more NO and stimulated nitrite-dependent vasodilation more potently than bHb despite both derivatives having similar initial rates for nitrite reduction and a more negative reduction potential in THb versus bHb. These data suggest that cross-linking of bovine hemoglobin in the T-state conformation leads to a more effective coupling of nitrite reduction to NO-formation. Our results support the model of allosteric regulation of nitrite reduction by deoxyhemoglobin and show that cross-linking hemoglobins in distinct quaternary states can generate products with increased NO yields from nitrite reduction that could be harnessed to promote NO-signaling in vivo.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures


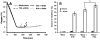
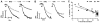
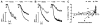
References
-
- Gladwin MT, Raat NJ, Shiva S, Dezfulian C, Hogg N, Kim-Shapiro DB, Patel RP. Nitrite as a vascular endocrine nitric oxide reservoir that contributes to hypoxic signaling, cytoprotection, and vasodilation. Am J Physiol Heart Circ Physiol. 2006;291:H2026–35. - PubMed
-
- Singel DJ, Stamler JS. Chemical physiology of blood flow regulation by red blood cells: the role of nitric oxide and S-nitrosohemoglobin. Annu Rev Physiol. 2005;67:99–145. - PubMed
-
- Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, Yang BK, Waclawiw MA, Zalos G, Xu X, Huang KT, Shields H, Kim-Shapiro DB, Schechter AN, Cannon RO, 3rd, Gladwin MT. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med. 2003;9:1498–505. - PubMed
-
- Kleinbongard P, Dejam A, Lauer T, Rassaf T, Schindler A, Picker O, Scheeren T, Godecke A, Schrader J, Schulz R, Heusch G, Schaub GA, Bryan NS, Feelisch M, Kelm M. Plasma nitrite reflects constitutive nitric oxide synthase activity in mammals. Free Radic Biol Med. 2003;35:790–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

