Heterodimers of the Arabidopsis transcription factors bZIP1 and bZIP53 reprogram amino acid metabolism during low energy stress
- PMID: 21278122
- PMCID: PMC3051235
- DOI: 10.1105/tpc.110.075390
Heterodimers of the Arabidopsis transcription factors bZIP1 and bZIP53 reprogram amino acid metabolism during low energy stress
Abstract
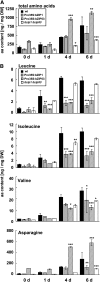
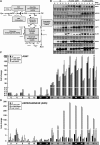
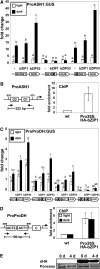
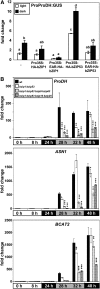
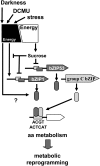
Control of energy homeostasis is crucial for plant survival, particularly under biotic or abiotic stress conditions. Energy deprivation induces dramatic reprogramming of transcription, facilitating metabolic adjustment. An in-depth knowledge of the corresponding regulatory networks would provide opportunities for the development of biotechnological strategies. Low energy stress activates the Arabidopsis thaliana group S1 basic leucine zipper transcription factors bZIP1 and bZIP53 by transcriptional and posttranscriptional mechanisms. Gain-of-function approaches define these bZIPs as crucial transcriptional regulators in Pro, Asn, and branched-chain amino acid metabolism. Whereas chromatin immunoprecipitation analyses confirm the direct binding of bZIP1 and bZIP53 to promoters of key metabolic genes, such as ASPARAGINE SYNTHETASE1 and PROLINE DEHYDROGENASE, the G-box, C-box, or ACT motifs (ACTCAT) have been defined as regulatory cis-elements in the starvation response. bZIP1 and bZIP53 were shown to specifically heterodimerize with group C bZIPs. Although single loss-of-function mutants did not affect starvation-induced transcription, quadruple mutants of group S1 and C bZIPs displayed a significant impairment. We therefore propose that bZIP1 and bZIP53 transduce low energy signals by heterodimerization with members of the partially redundant C/S1 bZIP factor network to reprogram primary metabolism in the starvation response.
Figures


References
-
- Alonso R., Oñate-Sánchez L., Weltmeier F., Ehlert A., Diaz I., Dietrich K., Vicente-Carbajosa J., Dröge-Laser W. (2009). A pivotal role of the basic leucine zipper transcription factor bZIP53 in the regulation of Arabidopsis seed maturation gene expression based on heterodimerization and protein complex formation. Plant Cell 21: 1747–1761 - PMC - PubMed
-
- Baena-González E., Rolland F., Thevelein J.M., Sheen J. (2007). A central integrator of transcription networks in plant stress and energy signalling. Nature 448: 938–942 - PubMed
-
- Bruneau L., Chapman R., Marsolais F. (2006). Co-occurrence of both L-asparaginase subtypes in Arabidopsis: At3g16150 encodes a K+-dependent L-asparaginase. Planta 224: 668–679 - PubMed
-
- Buchanan-Wollaston V., Page T., Harrison E., Breeze E., Lim P.O., Nam H.G., Lin J.F., Wu S.H., Swidzinski J., Ishizaki K., Leaver C.J. (2005). Comparative transcriptome analysis reveals significant differences in gene expression and signalling pathways between developmental and dark/starvation-induced senescence in Arabidopsis. Plant J. 42: 567–585 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

