Use of continuous glucose monitoring in subjects with type 1 diabetes on multiple daily injections versus continuous subcutaneous insulin infusion therapy: a prospective 6-month study
- PMID: 21278138
- PMCID: PMC3041183
- DOI: 10.2337/dc10-1852
Use of continuous glucose monitoring in subjects with type 1 diabetes on multiple daily injections versus continuous subcutaneous insulin infusion therapy: a prospective 6-month study
Abstract
Objective: To compare use of continuous glucose monitoring in subjects with type 1 diabetes on multiple daily injection (MDI) therapy versus continuous subcutaneous insulin infusion (CSII) therapy for 6 months.
Research design and methods: Sixty type 1 diabetic adults with similar baseline characteristics, using either MDI (n = 30) or CSII (n = 30) therapy, were enrolled in this 6-month prospective study. Subjects were instructed to wear the DexCom SevenPLUS continuous glucose monitor at all times throughout the study. All subjects were initially blinded from the continuous glucose monitoring (CGM) glucose data. After 4 weeks of blinded CGM use, the CGM was unblinded, making glucose data available to the patient. The CGM remained in the unblinded state for the remainder of the study (20 weeks). Clinic visits occurred every 4 weeks, at which time A1C values were collected and CGM data were downloaded.
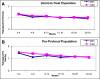
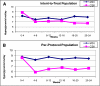
Results: Mean baseline (± SD) A1C was 7.61 (± 0.76) and 7.63 (± 0.68) for CSII and MDI, respectively (P > 0.05). Without any significant therapy change, A1C decrease at 12 weeks was similar in both groups (P = 0.03). When compared with the blinded phase, unblinded use of CGM was associated with similar but significant reductions in glycemic control and variability parameters. In addition, both therapy groups had similar changes in mean glucose and glucose variability indexes at 3 and 6 months (ITT analysis, P > 0.05). Predefined per protocol analysis (sensor use at least 6 days/week) showed greater improvement in time spent in target range glycemia, 3.9-10.0 mmol/L (70-180 mg/dL), in the CSII group.
Conclusions: We conclude that CGM provides similar benefits in glucose control for patients using MDI or CSII therapy.
Trial registration: ClinicalTrials.gov NCT01104142.
Figures
References
-
- Nathan DM, Cleary PA, Backlund JY, et al. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353:2643–2653 - PMC - PubMed
-
- Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 - PubMed
-
- Chase HP, Roberts MD, Wightman C, et al. Use of the GlucoWatch biographer in children with type 1 diabetes. Pediatrics 2003;111:790–794 - PubMed
-
- Ludvigsson J, Hanas R. Continuous subcutaneous glucose monitoring improved metabolic control in pediatric patients with type 1 diabetes: a controlled crossover study. Pediatrics 2003;111:933–938 - PubMed
-
- Tanenberg R, Bode B, Lane W, et al. Use of the continuous glucose monitoring system to guide therapy in patients with insulin-treated diabetes: a randomized controlled trial. Mayo Clin Proc 2004;79:1521–1526 - PubMed

