TEFM (c17orf42) is necessary for transcription of human mtDNA
- PMID: 21278163
- PMCID: PMC3105396
- DOI: 10.1093/nar/gkq1224
TEFM (c17orf42) is necessary for transcription of human mtDNA
Abstract
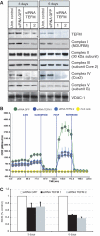
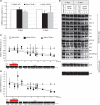
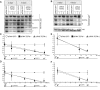
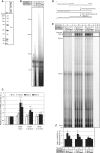
Here we show that c17orf42, hereafter TEFM (transcription elongation factor of mitochondria), makes a critical contribution to mitochondrial transcription. Inactivation of TEFM in cells by RNA interference results in respiratory incompetence owing to decreased levels of H- and L-strand promoter-distal mitochondrial transcripts. Affinity purification of TEFM from human mitochondria yielded a complex comprising mitochondrial transcripts, mitochondrial RNA polymerase (POLRMT), pentatricopeptide repeat domain 3 protein (PTCD3), and a putative DEAD-box RNA helicase, DHX30. After RNase treatment only POLRMT remained associated with TEFM, and in human cultured cells TEFM formed foci coincident with newly synthesized mitochondrial RNA. Based on deletion mutants, TEFM interacts with the catalytic region of POLRMT, and in vitro TEFM enhanced POLRMT processivity on ss- and dsDNA templates. TEFM contains two HhH motifs and a Ribonuclease H fold, similar to the nuclear transcription elongation regulator Spt6. These findings lead us to propose that TEFM is a mitochondrial transcription elongation factor.
Figures




References
-
- Forner F, Foster LJ, Campanaro S, Valle G, Mann M. Quantitative proteomic comparison of rat mitochondria from muscle, heart, and liver. Mol. Cell Proteomics. 2006;5:608–619. - PubMed
-
- Taylor SW, Fahy E, Zhang B, Glenn GM, Warnock DE, Wiley S, Murphy AN, Gaucher SP, Capaldi RA, Gibson BW, et al. Characterization of the human heart mitochondrial proteome. Nat. Biotechnol. 2003;21:281–286. - PubMed
-
- Calvo S, Jain M, Xie X, Sheth SA, Chang B, Goldberger OA, Spinazzola A, Zeviani M, Carr SA, Mootha VK. Systematic identification of human mitochondrial disease genes through integrative genomics. Nat. Genet. 2006;38:576–582. - PubMed
-
- Lopez MF, Kristal BS, Chernokalskaya E, Lazarev A, Shestopalov AI, Bogdanova A, Robinson M. High-throughput profiling of the mitochondrial proteome using affinity fractionation and automation. Electrophoresis. 2000;21:3427–3440. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

