Structural insights into membrane fusion at the endoplasmic reticulum
- PMID: 21278333
- PMCID: PMC3038745
- DOI: 10.1073/pnas.1019194108
Structural insights into membrane fusion at the endoplasmic reticulum
Conflict of interest statement
The authors declare no conflict of interest.
Figures
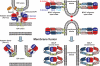
Comment on
-
Structural basis for the nucleotide-dependent dimerization of the large G protein atlastin-1/SPG3A.Proc Natl Acad Sci U S A. 2011 Feb 8;108(6):2216-21. doi: 10.1073/pnas.1012792108. Epub 2011 Jan 10. Proc Natl Acad Sci U S A. 2011. PMID: 21220294 Free PMC article.
References
-
- Orso G, et al. Homotypic fusion of ER membranes requires the dynamin-like GTPase atlastin. Nature. 2009;460:978–983. - PubMed
-
- Voeltz GK, Prinz WA, Shibata Y, Rist JM, Rapoport TA. A class of membrane proteins shaping the tubular endoplasmic reticulum. Cell. 2006;124:573–586. - PubMed
-
- Praefcke GJ, McMahon HT. The dynamin superfamily: Universal membrane tubulation and fission molecules? Nat Rev Mol Cell Biol. 2004;5:133–147. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

