CX3CR1+ lung mononuclear phagocytes spatially confined to the interstitium produce TNF-α and IL-6 and promote cigarette smoke-induced emphysema
- PMID: 21278339
- PMCID: PMC3912553
- DOI: 10.4049/jimmunol.1003221
CX3CR1+ lung mononuclear phagocytes spatially confined to the interstitium produce TNF-α and IL-6 and promote cigarette smoke-induced emphysema
Abstract
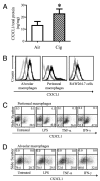
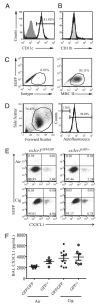
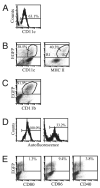
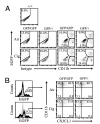
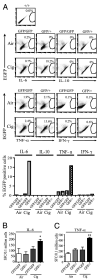
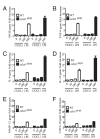
Increased numbers of macrophages are found in the lungs of smokers and those with chronic obstructive pulmonary disease. Experimental evidence shows the central role of macrophages in elaboration of inflammatory mediators such as TNF-α and the progression toward cigarette smoke-induced emphysema. We investigated the role of CX3CR1 in recruitment of mononuclear phagocytes, inflammatory cytokine responses, and tissue destruction in the lungs after cigarette smoke exposure. Using mice in which egfp is expressed at the locus of the cx3cr1 gene, we show that alveolar macrophages increased transmembrane ligand CX3CL1 expression and soluble CX3CL1 was detectable in the airspaces, but cx3cr1(GFP/GFP) and cx3cr1(GFP/+) mice failed to show recruitment of CX3CR1(+) cells into the airspaces with cigarette smoke. In contrast, cigarette smoke increased the accumulation of CX3CR1(+)CD11b(+) mononuclear phagocytes that were spatially confined to the lung interstitium and heterogenous in their expression of CD11c, MHC class II, and autofluorescent property. Although an intact CX3CL1-CX3CR1 pathway amplified the percentage of CX3CR1(+)CD11b(+) mononuclear phagocytes in the lungs, it was not essential for recruitment. Rather, functional CX3CR1 was required for a subset of tissue-bound mononuclear phagocytes to produce TNF-α and IL-6 in response to cigarette smoke, and the absence of functional CX3CR1 protected mice from developing tissue-destructive emphysema. Thus, CX3CR1(+) "tissue resident" mononuclear phagocytes initiate an innate immune response to cigarette smoke by producing TNF-α and IL-6 and are capable of promoting emphysema.
Figures

References
-
- Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet. 2007;370:765–773. - PubMed
-
- Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, Fukuchi Y, Jenkins C, Rodriguez-Roisin R, van Weel C, Zielinski J, Global Initiative for Chronic Obstructive Lung Disease Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am. J. Respir. Crit. Care Med. 2007;176:532–555. - PubMed
-
- Stockley RA, Mannino D, Barnes PJ. Burden and pathogenesis of chronic obstructive pulmonary disease. Proc. Am. Thorac. Soc. 2009;6:524–526. - PubMed
-
- Hautamaki RD, Kobayashi DK, Senior RM, Shapiro SD. Requirement for macrophage elastase for cigarette smoke-induced emphysema in mice. Science. 1997;277:2002–2004. - PubMed
-
- Churg A, Wang RD, Tai H, Wang X, Xie C, Dai J, Shapiro SD, Wright JL. Macrophage metalloelastase mediates acute cigarette smoke-induced inflammation via tumor necrosis factor-alpha release. Am. J. Respir. Crit. Care Med. 2003;167:1083–1089. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

