Breast cancer risk in relation to the interval between menopause and starting hormone therapy
- PMID: 21278356
- PMCID: PMC3039726
- DOI: 10.1093/jnci/djq527
Breast cancer risk in relation to the interval between menopause and starting hormone therapy
Abstract
Background: Although breast cancer risk is greater in users of estrogen-progestin than estrogen-only formulations of menopausal hormonal therapy, reports on their effects have been somewhat inconsistent. We investigated whether the timing of these therapies affected breast cancer incidence.
Methods: A total of 1,129,025 postmenopausal UK women provided prospective information on hormonal therapy use and other factors relevant for breast cancer risk. We used Cox regression to estimate adjusted relative risks (RRs) of breast cancer in hormonal therapy users vs never users and calculated standardized incidence rates. All statistical tests were two-sided.
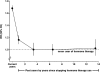
Results: During 4.05 million woman-years of follow-up, 15,759 incident breast cancers occurred, with 7107 in current users of hormonal therapy. Breast cancer incidence was increased in current users of hormonal therapy, returning to that of never users a few years after use had ceased. The relative risks for breast cancer in current users were greater if hormonal therapy was begun before or soon after menopause than after a longer gap (P(heterogeneity) < .001, for both estrogen-only and estrogen-progestin formulations). Among current users of estrogen-only formulations, there was little or no increase in risk if use began 5 years or more after menopause (RR = 1.05, 95% confidence interval [CI] = 0.89 to 1.24), but risk was statistically significantly increased if use began before or less than 5 years after menopause (RR = 1.43, 95% CI = 1.35 to 1.51). A similar pattern was observed among current users of estrogen-progestin formulations (RR = 1.53, 95% CI = 1.38 to 1.70, and RR = 2.04, 95% CI = 1.95 to 2.14, respectively). At 50-59 years of age, annual standardized incidence rates for breast cancer were 0.30% (95% CI = 0.29% to 0.31%) among never users of hormone therapy and 0.43% (95% CI = 0.42% to 0.45%) and 0.61% (95% CI = 0.59% to 0.64%), respectively, among current users of estrogen-only and estrogen-progestin formulations who began use less than 5 years after menopause.
Conclusions: There was substantial heterogeneity in breast cancer risk among current users of hormonal therapy. Risks were greater among users of estrogen-progestin than estrogen-only formulations and if hormonal therapy started at around the time of menopause than later.
Figures


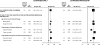


Comment in
-
The influence of time from menopause and mammography on hormone therapy-related breast cancer risk assessment.J Natl Cancer Inst. 2011 Feb 16;103(4):284-5. doi: 10.1093/jnci/djq561. Epub 2011 Jan 28. J Natl Cancer Inst. 2011. PMID: 21278357 No abstract available.
-
Re: Breast cancer risk in relation to the interval between menopause and starting hormone therapy.J Natl Cancer Inst. 2011 Jul 6;103(13):1069; reply 1069-70. doi: 10.1093/jnci/djr191. Epub 2011 Jun 23. J Natl Cancer Inst. 2011. PMID: 21700925 No abstract available.
-
Breast cancer risk and the interval between menopause and starting HT.Climacteric. 2011 Oct;14(5):598-9. doi: 10.3109/13697137.2011.608952. Climacteric. 2011. PMID: 21910672 No abstract available.
References
-
- International Agency for Research on Cancer. Monograph on the Evaluation of Carcinogenic Risks to Humans. Hormonal Contraception and Post-Menopausal Hormonal Therapy. Vol 72. Lyon, France: IARC Press; 1999.
-
- Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormone replacement therapy: collaborative reanalysis of data from 51 epidemiological studies of 52 705 women with breast cancer and 108 411 women without breast cancer. Lancet. 1997;350(9084):1047–1059. - PubMed
-
- Medicines and Healthcare products Regulatory Agency. UK Public Assessment Report. Hormone-Replacement Therapy: Safety Update. London, UK: MHRA; 2007. http://www.mhra.gov.uk/Safetyinformation/. Accessed April 27, 2009.
-
- The Women's Health Initiative Randomized Controlled Trial. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy. JAMA. 2004;291(14):1701–1712. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

