Single-molecule analysis of a molecular disassemblase reveals the mechanism of Hsc70-driven clathrin uncoating
- PMID: 21278753
- PMCID: PMC3056279
- DOI: 10.1038/nsmb.1985
Single-molecule analysis of a molecular disassemblase reveals the mechanism of Hsc70-driven clathrin uncoating
Abstract
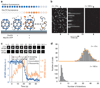
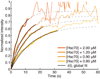
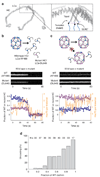
Heat shock cognate protein-70 (Hsc70) supports remodeling of protein complexes, such as disassembly of clathrin coats on endocytic coated vesicles. To understand how a simple ATP-driven molecular clamp catalyzes a large-scale disassembly reaction, we have used single-particle fluorescence imaging to track the dynamics of Hsc70 and its clathrin substrate in real time. Hsc70 accumulates to a critical level, determined by kinetic modeling to be one Hsc70 for every two functional attachment sites; rapid, all-or-none uncoating then ensues. We propose that Hsc70 traps conformational distortions, seen previously by cryo-EM, in the vicinity of each occupied site and that accumulation of local strains destabilizes the clathrin lattice. Capture of conformational fluctuations may be a general mechanism for chaperone-driven disassembly of protein complexes.
Figures
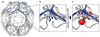




References
-
- Dodson M, McMacken R, Echols H. Specialized nucleoprotein structures at the origin of replication of bacteriophage lambda. Protein association and disassociation reactions responsible for localized initiation of replication. J Biol Chem. 1989;264:10719–10725. - PubMed
-
- Hartl FU, Hayer-Hartl M. Converging concepts of protein folding in vitro and in vivo. Nat Struct Mol Biol. 2009;16:574–581. - PubMed
-
- Rapoport TA. Protein translocation across the eukaryotic endoplasmic reticulum and bacterial plasma membranes. Nature. 2007;450:663–669. - PubMed
-
- Young JC, Barral JM, Ulrich Hartl F. More than folding: localized functions of cytosolic chaperones. Trends Biochem Sci. 2003;28:541–547. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

