PI3K inhibition results in enhanced HER signaling and acquired ERK dependency in HER2-overexpressing breast cancer
- PMID: 21278786
- PMCID: PMC3107390
- DOI: 10.1038/onc.2010.626
PI3K inhibition results in enhanced HER signaling and acquired ERK dependency in HER2-overexpressing breast cancer
Abstract
There is a strong rationale to therapeutically target the phosphatidylinositol 3-kinase/protein kinase B/mammalian target of rapamycin (PI3K/AKT/mTOR) pathway in breast cancer since it is highly deregulated in this disease and it also mediates resistance to anti-HER2 therapies. However, initial studies with rapalogs, allosteric inhibitors of mTORC1, have resulted in limited clinical efficacy probably due to the release of a negative regulatory feedback loop that triggers AKT and ERK signaling. Since activation of AKT occurs via PI3K, we decided to explore whether PI3K inhibitors prevent the activation of these compensatory pathways. Using HER2-overexpressing breast cancer cells as a model, we observed that PI3K inhibitors abolished AKT activation. However, PI3K inhibition resulted in a compensatory activation of the ERK signaling pathway. This enhanced ERK signaling occurred as a result of activation of HER family receptors as evidenced by induction of HER receptors dimerization and phosphorylation, increased expression of HER3 and binding of adaptor molecules to HER2 and HER3. The activation of ERK was prevented with either MEK inhibitors or anti-HER2 monoclonal antibodies and tyrosine kinase inhibitors. Combined administration of PI3K inhibitors with either HER2 or MEK inhibitors resulted in decreased proliferation, enhanced cell death and superior anti-tumor activity compared with single agent PI3K inhibitors. Our findings indicate that PI3K inhibition in HER2-overexpressing breast cancer activates a new compensatory pathway that results in ERK dependency. Combined anti-MEK or anti-HER2 therapy with PI3K inhibitors may be required in order to achieve optimal efficacy in HER2-overexpressing breast cancer. This approach warrants clinical evaluation.
Figures



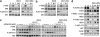
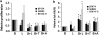

References
-
- André F, Campone M, Hurvitz SA, Vittori L, Pylvaenaeinen I, Sahmoud T, et al. 2008Multicenter phase I clinical trial of daily and weekly RAD001 in combination with weekly paclitaxel and trastuzumab in patients with HER2-overexpressing metastatic breast cancer with prior resistance to trastuzumab J Clin Oncol 26(Supplabstr 1003.
-
- Banerji U, Camidge DR, Verheul HM, Agarwal R, Sarker D, Kaye SB, et al. The first-in-human study of the hydrogen sulfate (Hyd-sulfate) capsule of the MEK1/2 inhibitor AZD6244 (ARRY-142886): a phase I open-label multicenter trial in patients with advanced cancer. Clin Cancer Res. 2010;16:1613–1623. - PubMed
-
- Baselga J, Semiglazov V, van Dam P, Manikhas A, Bellet M, Mayordomo J, et al. Phase II randomized study of neoadjuvant everolimus plus letrozole compared with placebo plus letrozole in patients with estrogen receptor-positive breast cancer. J Clin Oncol. 2009;27:2630–2637. - PubMed
-
- Baselga J, Swain SM. Novel anticancer targets: revisiting ERBB2 and discovering ERBB3. Nat Rev Cancer. 2009;9:463–475. - PubMed
-
- Bellacosa A, de Feo D, Godwin AK, Bell DW, Cheng JQ, Altomare DA, et al. Molecular alterations of the AKT2 oncogene in ovarian and breast carcinomas. Int J Cancer. 1995;64:280–285. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

