Neuropharmacology of Sleep and Wakefulness
- PMID: 21278831
- PMCID: PMC3026477
- DOI: 10.1016/j.jsmc.2010.08.003
Neuropharmacology of Sleep and Wakefulness
Abstract
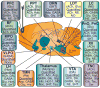
The development of sedative/hypnotic molecules has been empiric rather than rational. The empiric approach has produced clinically useful drugs but for no drug is the mechanism of action completely understood. All available sedative/hypnotic medications have unwanted side effects and none of these medications creates a sleep architecture that is identical to the architecture of naturally occurring sleep. This chapter reviews recent advances in research aiming to elucidate the neurochemical mechanisms regulating sleep and wakefulness. One promise of rational drug design is that understanding the mechanisms of sedative/hypnotic action will significantly enhance drug safety and efficacy.
Figures


References
-
- Watson CJ, Baghdoyan HA, Lydic R. A neurochemical perspective on states of consciousness. In: Hudetz AG, Pearce RA, editors. Suppressing the Mind: Anesthetic Modulation of Memory and Consciousness. New York: Springer/Humana Press; 2010. pp. 33–80.
-
- Steriade M, McCarley RW, editors. Brain Control of Wakefulness and Sleep. New York: Kluwer Academic/Plenum Publishers; 2005.
-
- McCarley RW. Neurobiology of REM and NREM sleep. Sleep Med. 2007;8:302–330. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

