Hsp-27 induction requires POU4F2/Brn-3b TF in doxorubicin-treated breast cancer cells, whereas phosphorylation alters its cellular localisation following drug treatment
- PMID: 21279488
- PMCID: PMC3118820
- DOI: 10.1007/s12192-011-0256-8
Hsp-27 induction requires POU4F2/Brn-3b TF in doxorubicin-treated breast cancer cells, whereas phosphorylation alters its cellular localisation following drug treatment
Abstract
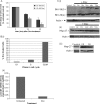
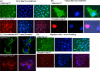
POU4F2/Brn-3b transcription factor (referred to as Brn-3b) is elevated in >60% of breast cancers and profoundly alters growth and behaviour of cancer cells by regulating distinct subsets of target genes. Previous studies showed that Brn-3b was required to maximally transactivate small heat shock protein, HSPB1/Hsp-27 (referred to as Hsp-27), and consequently, Brn-3b expression correlated well with Hsp27 levels in human breast biopsies. In these studies, we showed that Brn-3b is increased in MCF7 breast cancer cells that survive following treatment with chemotherapeutic drug doxorubicin (Dox) with concomitant increases in Hsp-27 expression. Targeting of Brn-3b using short interfering RNA reduced Hsp-27 in Dox-treated cells, suggesting that Brn-3b regulates Hsp-27 expression under these conditions. Wound healing assays showed increased Brn-3b in Dox-treated migratory cells that also express Hsp-27. Interestingly, Hsp-27 phosphorylation and cellular localisation are also significantly altered at different times following Dox treatment. Thus, phospho-Hsp-27 (p-Hsp27) protein displayed widespread distribution after 24 hrs of Dox treatment but was restricted to the nucleus after 5 days. However, in drug-resistant cells (grown in Dox for > 1 month), p-Hsp-27 was excluded from nuclei and most of the cytoplasm and appeared to be associated with the cell membrane. Studies to determine how this protein promotes survival and migration in breast cancer cells showed that the protective effects were conferred by unphosphorylated Hsp-27 protein. Thus, complex and dynamic mechanisms underlie effects of Hsp-27 protein in breast cancer cells following treatment with chemotherapeutic drugs such as Dox, and this may contribute to invasiveness and drug resistance following chemotherapy.
Figures




Similar articles
-
Proliferation-associated POU4F2/Brn-3b transcription factor expression is regulated by oestrogen through ERα and growth factors via MAPK pathway.Breast Cancer Res. 2011 Jan 17;13(1):R5. doi: 10.1186/bcr2809. Breast Cancer Res. 2011. PMID: 21241485 Free PMC article.
-
Expression of the Brn-3b transcription factor correlates with expression of HSP-27 in breast cancer biopsies and is required for maximal activation of the HSP-27 promoter.Cancer Res. 2005 Apr 15;65(8):3072-80. doi: 10.1158/0008-5472.CAN-04-2865. Cancer Res. 2005. PMID: 15833836
-
Cardiac expression of Brn-3a and Brn-3b POU transcription factors and regulation of Hsp27 gene expression.Cell Stress Chaperones. 2008 Sep;13(3):297-312. doi: 10.1007/s12192-008-0028-2. Epub 2008 Mar 27. Cell Stress Chaperones. 2008. PMID: 18368538 Free PMC article.
-
Targeting Brn-3b in breast cancer therapy.Expert Opin Ther Targets. 2006 Feb;10(1):15-25. doi: 10.1517/14728222.10.1.15. Expert Opin Ther Targets. 2006. PMID: 16441225 Review.
-
Linking metabolic dysfunction with cardiovascular diseases: Brn-3b/POU4F2 transcription factor in cardiometabolic tissues in health and disease.Cell Death Dis. 2021 Mar 12;12(3):267. doi: 10.1038/s41419-021-03551-9. Cell Death Dis. 2021. PMID: 33712567 Free PMC article. Review.
Cited by
-
Heat shock proteins and heat shock factor 1 in carcinogenesis and tumor development: an update.Arch Toxicol. 2013 Jan;87(1):19-48. doi: 10.1007/s00204-012-0918-z. Epub 2012 Aug 11. Arch Toxicol. 2013. PMID: 22885793 Free PMC article. Review.
-
SILAC-based phosphoproteomics reveals an inhibitory role of KSR1 in p53 transcriptional activity via modulation of DBC1.Br J Cancer. 2013 Nov 12;109(10):2675-84. doi: 10.1038/bjc.2013.628. Epub 2013 Oct 15. Br J Cancer. 2013. PMID: 24129246 Free PMC article.
-
Essential but partially redundant roles for POU4F1/Brn-3a and POU4F2/Brn-3b transcription factors in the developing heart.Cell Death Dis. 2017 Jun 8;8(6):e2861. doi: 10.1038/cddis.2017.185. Cell Death Dis. 2017. PMID: 28594399 Free PMC article.
-
HspB1, HspB5 and HspB4 in Human Cancers: Potent Oncogenic Role of Some of Their Client Proteins.Cancers (Basel). 2014 Feb 7;6(1):333-65. doi: 10.3390/cancers6010333. Cancers (Basel). 2014. PMID: 24514166 Free PMC article.
-
Somatic Genomics and Clinical Features of Lung Adenocarcinoma: A Retrospective Study.PLoS Med. 2016 Dec 6;13(12):e1002162. doi: 10.1371/journal.pmed.1002162. eCollection 2016 Dec. PLoS Med. 2016. PMID: 27923066 Free PMC article.
References
-
- Benndorf R, Hayess K, Ryazantsev S, Wieske M, Behlke J, Lutsch G. Phosphorylation and supramolecular organization of murine small heat shock protein HSP25 abolish its actin polymerization-inhibiting activity. J Biol Chem. 1994;269:20780–20784. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

