Reassessment of the structural basis of the ascending arousal system
- PMID: 21280045
- PMCID: PMC3119596
- DOI: 10.1002/cne.22559
Reassessment of the structural basis of the ascending arousal system
Erratum in
- J Comp Neurol. 2011 Dec 15;519(18):3817. Fuller, Patrick [corrected to Fuller, Patrick M]
Abstract
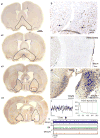
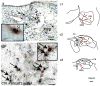
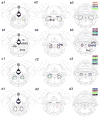
The "ascending reticular activating system" theory proposed that neurons in the upper brainstem reticular formation projected to forebrain targets that promoted wakefulness. More recent formulations have emphasized that most neurons at the pontomesencephalic junction that participate in these pathways are actually in monoaminergic and cholinergic cell groups. However, cell-specific lesions of these cell groups have never been able to reproduce the deep coma seen after acute paramedian midbrain lesions that transect ascending axons at the caudal midbrain level. To determine whether the cortical afferents from the thalamus or the basal forebrain were more important in maintaining arousal, we first placed large cell-body-specific lesions in these targets. Surprisingly, extensive thalamic lesions had little effect on electroencephalographic (EEG) or behavioral measures of wakefulness or on c-Fos expression by cortical neurons during wakefulness. In contrast, animals with large basal forebrain lesions were behaviorally unresponsive and had a monotonous sub-1-Hz EEG, and little cortical c-Fos expression during continuous gentle handling. We then retrogradely labeled inputs to the basal forebrain from the upper brainstem, and found a substantial input from glutamatergic neurons in the parabrachial nucleus and adjacent precoeruleus area. Cell-specific lesions of the parabrachial-precoeruleus complex produced behavioral unresponsiveness, a monotonous sub-1-Hz cortical EEG, and loss of cortical c-Fos expression during gentle handling. These experiments indicate that in rats the reticulo-thalamo-cortical pathway may play a very limited role in behavioral or electrocortical arousal, whereas the projection from the parabrachial nucleus and precoeruleus region, relayed by the basal forebrain to the cerebral cortex, may be critical for this process.
Copyright © 2010 Wiley-Liss, Inc.
Figures









References
-
- Adametz JH. Rate of recovery of functioning in cats with rostral reticular lesions; an experimental study. J Neurosurg. 1959;16:85–97. - PubMed
-
- Adams JH, Graham DI, Murray LS, Scott G. Diffuse axonal injury due to nonmissile head injury in humans: an analysis of 45 cases. Ann Neurol. 1982;12:557–63. - PubMed
-
- Batini C, Moruzzi G, Palestini M, Rossi GF, Zanchetti A. Effects of complete pontine transections on the sleep-wakefulness rhythm: the midpontine pretri-geminal preparation. Arch Ital Biol. 1959;97:1–12.
-
- Berntson GG, Shafi R, Sarter M. Specific contributions of the basal forebrain corticopetal cholinergic system to electroencephalographic activity and sleep/waking behaviour. Eur J Neurosci. 2002;16:2453–61. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

