Fingolimod provides long-term protection in rodent models of cerebral ischemia
- PMID: 21280082
- PMCID: PMC3200194
- DOI: 10.1002/ana.22186
Fingolimod provides long-term protection in rodent models of cerebral ischemia
Abstract
Objective: The sphingosine-1-phosphate (S1P) receptor agonist fingolimod (FTY720), that has shown efficacy in advanced multiple sclerosis clinical trials, decreases reperfusion injury in heart, liver, and kidney. We therefore tested the therapeutic effects of fingolimod in several rodent models of focal cerebral ischemia. To assess the translational significance of these findings, we asked whether fingolimod improved long-term behavioral outcomes, whether delayed treatment was still effective, and whether neuroprotection can be obtained in a second species.
Methods: We used rodent models of middle cerebral artery occlusion and cell-culture models of neurotoxicity and inflammation to examine the therapeutic potential and mechanisms of neuroprotection by fingolimod.
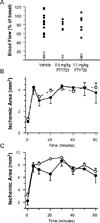
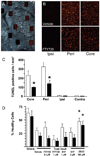
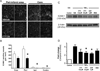
Results: In a transient mouse model, fingolimod reduced infarct size, neurological deficit, edema, and the number of dying cells in the core and periinfarct area. Neuroprotection was accompanied by decreased inflammation, as fingolimod-treated mice had fewer activated neutrophils, microglia/macrophages, and intercellular adhesion molecule-1 (ICAM-1)-positive blood vessels. Fingolimod-treated mice showed a smaller infarct and performed better in behavioral tests up to 15 days after ischemia. Reduced infarct was observed in a permanent model even when mice were treated 4 hours after ischemic onset. Fingolimod also decreased infarct size in a rat model of focal ischemia. Fingolimod did not protect primary neurons against glutamate excitotoxicity or hydrogen peroxide, but decreased ICAM-1 expression in brain endothelial cells stimulated by tumor necrosis factor alpha.
Interpretation: These findings suggest that anti-inflammatory mechanisms, and possibly vasculoprotection, rather than direct effects on neurons, underlie the beneficial effects of fingolimod after stroke. S1P receptors are a highly promising target in stroke treatment.
Copyright © 2010 American Neurological Association.
Figures



References
-
- Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22:391–397. - PubMed
-
- Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol. 2008;9:139–150. - PubMed
-
- Karliner JS, Honbo N, Summers K, et al. The lysophospholipids sphingosine-1-phosphate and lysophosphatidic acid enhance survival during hypoxia in neonatal rat cardiac myocytes. J Mol Cell Cardiol. 2001;33:1713–1717. - PubMed
-
- Jin ZQ, Zhou HZ, Zhu P, et al. Cardioprotection mediated by sphingosine-1-phosphate and ganglioside GM-1 in wild-type and PKC epsilon knockout mouse hearts. Am J Physiol Heart Circ Physiol. 2002;282:H1970–H1977. - PubMed
-
- Adachi K, Kohara T, Nakao N, et al. Design, synthesis, and structure-activity relationships of 2-substituted-2-amino-1,3-propanediols: Discovery of a novel immunosuppressant, FTY720. Bioorg Med Chem Lett. 1995;5:853–856.
Publication types
MeSH terms
Substances
Grants and funding
- P01 NS010828/NS/NINDS NIH HHS/United States
- P01 HL048743/HL/NHLBI NIH HHS/United States
- R37NS37074/NS/NINDS NIH HHS/United States
- R37 NS037074/NS/NINDS NIH HHS/United States
- HL052233/HL/NHLBI NIH HHS/United States
- R01NS049263/NS/NINDS NIH HHS/United States
- R01 NS053560/NS/NINDS NIH HHS/United States
- R01 HL052233/HL/NHLBI NIH HHS/United States
- R01 NS070001/NS/NINDS NIH HHS/United States
- P01 NS055104/NS/NINDS NIH HHS/United States
- R01 HL080187/HL/NHLBI NIH HHS/United States
- R01 DK085006/DK/NIDDK NIH HHS/United States
- R01NS53560/NS/NINDS NIH HHS/United States
- R01 NS049263/NS/NINDS NIH HHS/United States
- P01NS55104/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

