Prospective identification and isolation of enteric nervous system progenitors using Sox2
- PMID: 21280162
- PMCID: PMC3059409
- DOI: 10.1002/stem.557
Prospective identification and isolation of enteric nervous system progenitors using Sox2
Abstract
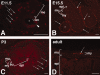
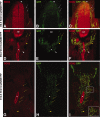
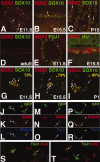
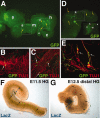
The capacity to identify and isolate lineage-specific progenitor cells from developing and mature tissues would enable the development of cell replacement therapies for disease treatment. The enteric nervous system (ENS) regulates important gut functions, including controlling peristaltic muscular contractions, and consists of interconnected ganglia containing neurons and glial cells. Hirschsprung's disease (HSCR), one of the most common and best understood diseases affecting the ENS, is characterized by absence of enteric ganglia from the distal gut due to defects in gut colonization by neural crest progenitor cells and is an excellent candidate for future cell replacement therapies. Our previous microarray experiments identified the neural progenitor and stem cell marker SRY-related homoebox transcription factor 2 (Sox2) as expressed in the embryonic ENS. We now show that Sox2 is expressed in the ENS from embryonic to adult stages and constitutes a novel marker of ENS progenitor cells and their glial cell derivatives. We also show that Sox2 expression overlaps significantly with SOX10, a well-established marker of ENS progenitors and enteric glial cells. We have developed a strategy to select cells expressing Sox2, by using G418 selection on cultured gut cells derived from Sox2(βgeo/+) mouse embryos, thus allowing substantial enrichment and expansion of neomycin-resistant Sox2-expressing cells. Sox2(βgeo) cell cultures are enriched for ENS progenitors. Following transplantation into embryonic mouse gut, Sox2(βgeo) cells migrate, differentiate, and colocalize with the endogenous ENS plexus. Our studies will facilitate development of cell replacement strategies in animal models, critical to develop human cell replacement therapies for HSCR.
Copyright © 2010 AlphaMed Press.
Figures



References
-
- Heanue TA, Pachnis V. Enteric nervous system development and Hirschsprung's disease: Advances in genetic and stem cell studies. Nat Rev Neurosci. 2007;8:466–479. - PubMed
-
- Newgreen D, Young HM. Enteric nervous system: Development and developmental disturbances—Part 2. Pediatr Dev Pathol. 2002;5:329–349. - PubMed
-
- Newgreen D, Young HM. Enteric nervous system: Development and developmental disturbances—Part 1. Pediatr Dev Pathol. 2002;5:224–247. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

