Bending and puncturing the influenza lipid envelope
- PMID: 21281578
- PMCID: PMC3030173
- DOI: 10.1016/j.bpj.2010.12.3701
Bending and puncturing the influenza lipid envelope
Abstract
Lysosomes, enveloped viruses, as well as synaptic and secretory vesicles are all examples of natural nanocontainers (diameter ≈ 100 nm) which specifically rely on their lipid bilayer to protect and exchange their contents with the cell. We have applied methods primarily based on atomic force microscopy and finite element modeling that allow precise investigation of the mechanical properties of the influenza virus lipid envelope. The mechanical properties of small, spherical vesicles made from PR8 influenza lipids were probed by an atomic force microscopy tip applying forces up to 0.2 nN, which led to an elastic deformation up to 20%, on average. The liposome deformation was modeled using finite element methods to extract the lipid bilayer elastic properties. We found that influenza liposomes were softer than what would be expected for a gel phase bilayer and highly deformable: Consistent with previous suggestion that influenza lipids do not undergo a major phase transition, we observe that the stiffness of influenza liposomes increases gradually and weakly (within one order of magnitude) with temperature. Surprisingly, influenza liposomes were, in most cases, able to withstand wall-to-wall deformation, and forces >1 nN were generally required to puncture the influenza envelope, which is similar to viral protein shells. Hence, the choice of a highly flexible lipid envelope may provide as efficient a protection for a viral genome as a stiff protein shell.
Copyright © 2011 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures
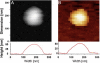
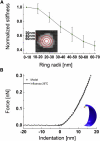



References
-
- Lipowsky R., Sackmann E., editors. Structure and Dynamics of Membranes. Elsevier; Dordrecht, The Netherlands: 1995.
-
- Lentz B.R., Carpenter T.J., Alford D.R. Spontaneous fusion of phosphatidylcholine small unilamellar vesicles in the fluid phase. Biochemistry. 1987;26:5389–5397. - PubMed
-
- Scheiffele P., Rietveld A., Simons K. Influenza viruses select ordered lipid domains during budding from the plasma membrane. J. Biol. Chem. 1999;274:2038–2044. - PubMed
-
- Polozov I.V., Bezrukov L., Zimmerberg J. Progressive ordering with decreasing temperature of the phospholipids of influenza virus. Nat. Chem. Biol. 2008;4:248–255. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

