Six1 regulates Grem1 expression in the metanephric mesenchyme to initiate branching morphogenesis
- PMID: 21281623
- PMCID: PMC3113441
- DOI: 10.1016/j.ydbio.2011.01.027
Six1 regulates Grem1 expression in the metanephric mesenchyme to initiate branching morphogenesis
Abstract
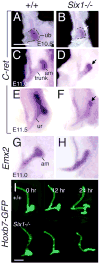
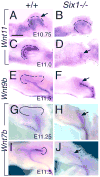
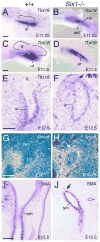
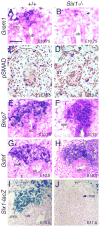
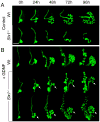
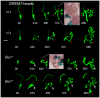
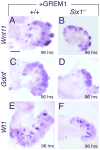
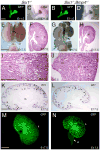
Urinary tract morphogenesis requires subdivision of the ureteric bud (UB) into the intra-renal collecting system and the extra-renal ureter, by responding to signals in its surrounding mesenchyme. BMP4 is a mesenchymal regulator promoting ureter development, while GREM1 is necessary to negatively regulate BMP4 activity to induce UB branching. However, the mechanisms that regulate the GREM1-BMP4 signaling are unknown. Previous studies have shown that Six1-deficient mice lack kidneys, but form ureters. Here, we show that the tip cells of Six1(-/-) UB fail to form an ampulla for branching. Instead, the UB elongates within Tbx18- and Bmp4-expressing mesenchyme. We find that the expression of Grem1 in the metanephric mesenchyme (MM) is Six1-dependent. Treatment of Six1(-/-) kidney rudiments with GREM1 protein restores ampulla formation and branching morphogenesis. Furthermore, we demonstrate that genetic reduction of BMP4 levels in Six1(-/-) (Six1(-/-); Bmp4(+/-)) embryos restores urinary tract morphogenesis and kidney formation. This study uncovers an essential function for Six1 in the MM as an upstream regulator of Grem1 in initiating branching morphogenesis.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Reduction of BMP4 activity by gremlin 1 enables ureteric bud outgrowth and GDNF/WNT11 feedback signalling during kidney branching morphogenesis.Development. 2007 Jul;134(13):2397-405. doi: 10.1242/dev.02861. Epub 2007 May 23. Development. 2007. PMID: 17522159
-
SIX1 acts synergistically with TBX18 in mediating ureteral smooth muscle formation.Development. 2010 Mar;137(5):755-65. doi: 10.1242/dev.045757. Epub 2010 Jan 28. Development. 2010. PMID: 20110314 Free PMC article.
-
Hs2st mediated kidney mesenchyme induction regulates early ureteric bud branching.Dev Biol. 2010 Mar 15;339(2):354-65. doi: 10.1016/j.ydbio.2009.12.033. Epub 2010 Jan 6. Dev Biol. 2010. PMID: 20059993 Free PMC article.
-
Genetic controls and cellular behaviors in branching morphogenesis of the renal collecting system.Wiley Interdiscip Rev Dev Biol. 2012 Sep-Oct;1(5):693-713. doi: 10.1002/wdev.52. Wiley Interdiscip Rev Dev Biol. 2012. PMID: 22942910 Free PMC article. Review.
-
GDNF/Ret signaling and the development of the kidney.Bioessays. 2006 Feb;28(2):117-27. doi: 10.1002/bies.20357. Bioessays. 2006. PMID: 16435290 Review.
Cited by
-
Lower urinary tract development and disease.Wiley Interdiscip Rev Syst Biol Med. 2013 May-Jun;5(3):307-42. doi: 10.1002/wsbm.1212. Epub 2013 Feb 13. Wiley Interdiscip Rev Syst Biol Med. 2013. PMID: 23408557 Free PMC article. Review.
-
Study of cellular heterogeneity and differential dynamics of autophagy in human embryonic kidney development by single-cell RNA sequencing.Cancer Cell Int. 2021 Aug 30;21(1):460. doi: 10.1186/s12935-021-02154-w. Cancer Cell Int. 2021. PMID: 34461918 Free PMC article.
-
The genetics and pathogenesis of CAKUT.Nat Rev Nephrol. 2023 Nov;19(11):709-720. doi: 10.1038/s41581-023-00742-9. Epub 2023 Jul 31. Nat Rev Nephrol. 2023. PMID: 37524861 Review.
-
Disruption of Gen1 Causes Congenital Anomalies of the Kidney and Urinary Tract in Mice.Int J Biol Sci. 2018 Jan 1;14(1):10-20. doi: 10.7150/ijbs.22768. eCollection 2018. Int J Biol Sci. 2018. PMID: 29483821 Free PMC article.
-
Diverse feather shape evolution enabled by coupling anisotropic signalling modules with self-organizing branching programme.Nat Commun. 2017 Jan 20;8:ncomms14139. doi: 10.1038/ncomms14139. Nat Commun. 2017. PMID: 28106042 Free PMC article.
References
-
- Airik R, Kispert A. Down the tube of obstructive nephropathies: the importance of tissue interactions during ureter development. Kidney Int. 2007;72:1459–67. - PubMed
-
- al-Awqati Q, Goldberg MR. Architectural patterns in branching morphogenesis in the kidney. Kidney Int. 1998;54:1832–42. - PubMed
-
- Brenner-Anantharam A, Cebrian C, Guillaume R, Hurtado R, Sun TT, Herzlinger D. Tailbud-derived mesenchyme promotes urinary tract segmentation via BMP4 signaling. Development. 2007;134:1967–75. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

