Genistein effects on stromal cells determines epithelial proliferation in endometrial co-cultures
- PMID: 21281625
- PMCID: PMC3092029
- DOI: 10.1016/j.yexmp.2011.01.006
Genistein effects on stromal cells determines epithelial proliferation in endometrial co-cultures
Abstract
Background: Estrogen is the leading etiologic factor for endometrial cancer. Estrogen-induced proliferation of endometrial epithelial cells normally requires paracrine growth factors produced by stromal cells. Epidemiologic evidence indicates that dietary soy prevents endometrial cancer, and implicates the phytoestrogen genistein in this effect. However, results from previous studies are conflicting regarding the effects of genistein on hormone responsive cancers.
Methods: The effects of estrogen and genistein on proliferation of Ishikawa (IK) endometrial adenocarcinoma cells were examined in co-cultures of IK cells with endometrial stromal cells, recapitulating the heterotypic cell-to-cell interactions observed in vivo. The roles of estrogen receptor (ER)α and ERβ were evaluated using ERα and ERβ specific agonists. ER activation and cell proliferation in the IK epithelial cells were determined by alkaline phosphatase assay and Coulter counter enumeration, respectively.
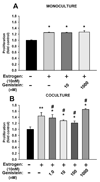
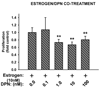
Results: Both estrogen and genistein increased estrogen receptor-induced gene activity in IK cells over a range of concentrations. Estrogen alone but not genistein increased IK proliferation in co-cultures. When primed by estrogen treatment, increasing concentrations of genistein produced a biphasic effect on IK proliferation: nM concentrations inhibited estrogen-induced proliferation while μM concentrations increased proliferation. Studies with an ERβ-specific agonist produced similar results. Genistein did not influence the effects of estrogen on IK proliferation in monoculture.
Conclusions: Our study indicates that nutritionally relevant concentrations (nM) of genistein inhibit the proliferative effects of estrogen on endometrial adenocarcinoma cells presumably through activation of stromal cell ERβ. We believe that sub-micromolar concentrations of genistein may represent a novel adjuvant for endometrial cancer treatment and prevention.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
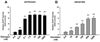

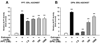

Similar articles
-
Stimulation of endometrial glandular cells with genistein and daidzein and their effects on ERalpha- and ERbeta-mRNA and protein expresion.Anticancer Res. 2005 May-Jun;25(3A):1713-8. Anticancer Res. 2005. PMID: 16033089
-
Effect of normal endometrial stroma on growth and differentiation in Ishikawa endometrial adenocarcinoma cells.Cancer Res. 2002 Jan 1;62(1):79-88. Cancer Res. 2002. PMID: 11782363
-
[Regulation of genistein on the levels of ERalpha, ERbeta mRNA in uterine endometrial cancer cells].Beijing Da Xue Xue Bao Yi Xue Ban. 2005 Jun 18;37(3):278-80. Beijing Da Xue Xue Bao Yi Xue Ban. 2005. PMID: 15968319 Chinese.
-
Acetaminophen exhibits weak antiestrogenic activity in human endometrial adenocarcinoma (Ishikawa) cells.Toxicol Sci. 2003 Mar;72(1):57-65. doi: 10.1093/toxsci/kfg005. Toxicol Sci. 2003. PMID: 12604834
-
Estrogen receptor β: the guardian of the endometrium.Hum Reprod Update. 2015 Mar-Apr;21(2):174-93. doi: 10.1093/humupd/dmu053. Epub 2014 Oct 10. Hum Reprod Update. 2015. PMID: 25305176 Review.
Cited by
-
Gene expression analysis of in vitro cocultures to study interactions between breast epithelium and stroma.J Biomed Biotechnol. 2011;2011:520987. doi: 10.1155/2011/520987. Epub 2011 Dec 13. J Biomed Biotechnol. 2011. PMID: 22203785 Free PMC article. Review.
-
Diverse effects of phytoestrogens on the reproductive performance: cow as a model.Int J Endocrinol. 2013;2013:650984. doi: 10.1155/2013/650984. Epub 2013 Apr 23. Int J Endocrinol. 2013. PMID: 23710176 Free PMC article.
-
Novasoy and genistein inhibit endometrial cancer cell proliferation through disruption of the AKT/mTOR and MAPK signaling pathways.Am J Transl Res. 2018 Mar 15;10(3):784-795. eCollection 2018. Am J Transl Res. 2018. PMID: 29636868 Free PMC article.
-
Influence of AKT on progesterone action in endometrial diseases.Biol Reprod. 2014 Sep;91(3):63. doi: 10.1095/biolreprod.114.119255. Epub 2014 Aug 6. Biol Reprod. 2014. PMID: 25100707 Free PMC article. Review.
-
Progesterone action in endometrial cancer, endometriosis, uterine fibroids, and breast cancer.Endocr Rev. 2013 Feb;34(1):130-62. doi: 10.1210/er.2012-1043. Epub 2013 Jan 9. Endocr Rev. 2013. PMID: 23303565 Free PMC article. Review.
References
-
- Amant F, Moerman P, Neven P, Timmerman D, Van Limbergen E, Vergote I. Endometrial cancer. Lancet. 2005;366:491–505. - PubMed
-
- American Cancer Society. Cancer Facts & Figures 2010. Atlanta, GA: American Cancer Society; 2010.
-
- Arnold JT, Kaufman DG, Seppala M, Lessey BA. Endometrial stromal cells regulate epithelial cell growth in vitro: a new co-culture model. Hum. Reprod. 2001;16:836–845. - PubMed
-
- Arnold JT, Lessey BA, Seppala M, Kaufman DG. Effect of normal endometrial stroma on growth and differentiation in Ishikawa endometrial adenocarcinoma cells. Cancer Res. 2002;62:79–88. - PubMed
-
- Barbier CS, Becker KA, Troester MA, Kaufman DG. Expression of exogenous human telomerase in cultures of endometrial stromal cells does not alter their hormone responsiveness. Biol. Reprod. 2005;73:106–114. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 ES010126/ES/NIEHS NIH HHS/United States
- CA096960/CA/NCI NIH HHS/United States
- T32 ES007017/ES/NIEHS NIH HHS/United States
- L60 MD002928/MD/NIMHD NIH HHS/United States
- 1 L60 MD002928-01/MD/NIMHD NIH HHS/United States
- F31-GM073349/GM/NIGMS NIH HHS/United States
- R01 CA096960/CA/NCI NIH HHS/United States
- T32 CA072319/CA/NCI NIH HHS/United States
- T32-ES007017/ES/NIEHS NIH HHS/United States
- P30-ES010126/ES/NIEHS NIH HHS/United States
- T32-CA072319/CA/NCI NIH HHS/United States
- F31 GM073349/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous

