Attenuation of the lysosomal death pathway by lysosomal cholesterol accumulation
- PMID: 21281795
- PMCID: PMC3069902
- DOI: 10.1016/j.ajpath.2010.10.030
Attenuation of the lysosomal death pathway by lysosomal cholesterol accumulation
Abstract
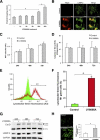
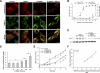
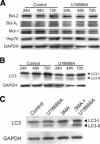
In the past decade, lysosomal membrane permeabilization (LMP) has emerged as a significant component of cell death signaling. The mechanisms by which lysosomal stability is regulated are not yet fully understood, but changes in the lysosomal membrane lipid composition have been suggested to be involved. Our aim was to investigate the importance of cholesterol in the regulation of lysosomal membrane permeability and its potential impact on apoptosis. Treatment of normal human fibroblasts with U18666A, an amphiphilic drug that inhibits cholesterol transport and causes accumulation of cholesterol in lysosomes, rescued cells from lysosome-dependent cell death induced by the lysosomotropic detergent O-methyl-serine dodecylamide hydrochloride (MSDH), staurosporine (STS), or cisplatin. LMP was decreased by pretreating cells with U18666A, and there was a linear relationship between the cholesterol content of lysosomes and their resistance to permeabilization induced by MSDH. U18666A did not induce changes in expression or localization of 70-kDa heat shock proteins (Hsp70) or antiapoptotic Bcl-2 proteins known to protect the lysosomal membrane. Induction of autophagy also was excluded as a contributor to the protective mechanism. By using Chinese hamster ovary (CHO) cells with lysosomal cholesterol overload due to a mutation in the cholesterol transporting protein Niemann-Pick type C1 (NPC1), the relationship between lysosomal cholesterol accumulation and protection from lysosome-dependent cell death was confirmed. Cholesterol accumulation in lysosomes attenuates apoptosis by increasing lysosomal membrane stability.
Copyright © 2011 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Boya P., Kroemer G. Lysosomal membrane permeabilization in cell death. Oncogene. 2008;27:6434–6451. - PubMed
-
- Firestone R.A., Pisano J.M., Bonney R.J. Lysosomotropic agents. 1. Synthesis and cytotoxic action of lysosomotropic detergents. J Med Chem. 1979;22:1130–1133. - PubMed
-
- Li W., Yuan X., Nordgren G., Dalen H., Dubowchik G.M., Firestone R.A., Brunk U.T. Induction of cell death by the lysosomotropic detergent MSDH. FEBS Lett. 2000;470:35–39. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

