Mast cells are an essential component of human radiation proctitis and contribute to experimental colorectal damage in mice
- PMID: 21281796
- PMCID: PMC3069878
- DOI: 10.1016/j.ajpath.2010.10.003
Mast cells are an essential component of human radiation proctitis and contribute to experimental colorectal damage in mice
Abstract
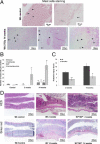
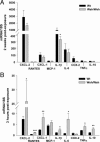
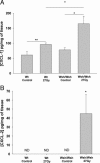
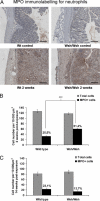
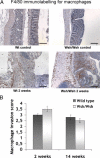
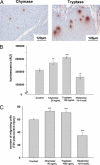
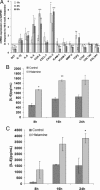
Radiation proctitis is characterized by mucosal inflammation followed by adverse chronic tissue remodeling and is associated with substantial morbidity and mortality. Mast cell hyperplasia has been associated with diseases characterized by pathological tissue remodeling and fibrosis. Rectal tissue from patients treated with radiotherapy shows mast cell hyperplasia and activation, suggesting that these cells play a role in the development of radiation-induced sequelae. To investigate the role of mast cells in radiation damage, experimental radiation proctitis was induced in a mast cell-deficient (W(sh)/W(sh)) mouse model. The colon and rectum of W(sh)/W(sh) and wild-type mice were exposed to 27-Gy single-dose irradiation and studied after 2 and 14 weeks. Irradiated rodent rectum showed mast cell hyperplasia. W(sh)/W(sh) mice developed less acute and chronic rectal radiation damage than their control littermates. Tissue protection was associated with increased tissue neutrophil influx and expression of several inflammatory mediators immediately after radiation exposure. It was further demonstrated that mast cell chymase, tryptase, and histamine could change human muscularis propria smooth muscle cells into a migrating/proliferating and proinflammatory phenotype. These data show that mast cells have deleterious effects on both acute and chronic radiation proctitis, possibly by limiting acute tissue neutrophil influx and by favoring phenotypic orientation of smooth muscle cells, thus making them active participants in the radiation-induced inflammatory process and dystrophy of the rectal wall.
Copyright © 2011 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Bentzen S.M., Dörr W., Anscher M.S., Denham J.W., Hauer-Jensen M., Marks L.B., Williams J. Normal tissue effects: reporting and analysis. Semin Radiat Oncol. 2003;13:189–202. - PubMed
-
- Andreyev H.J. Gastrointestinal problems after pelvic radiotherapy: the past, the present and the future. Clin Oncol (R Coll Radiol) 2007;19:790–799. - PubMed
-
- Andreyev J. Gastrointestinal symptoms after pelvic radiotherapy: a new understanding to improve management of symptomatic patients. Lancet Oncol. 2007;8:1007–1017. - PubMed
-
- Hovdenak N., Fajardo L.F., Hauer-Jensen M. Acute radiation proctitis: a sequential clinicopathologic study during pelvic radiotherapy. Int J Radiat Oncol Biol Phys. 2000;48:1111–1117. - PubMed
-
- Garg A.K., Mai W.Y., McGary J.E., Grant W.H., 3rd, Butler E.B., Teh B.S. Radiation proctopathy in the treatment of prostate cancer. Int J Radiat Oncol Biol Phys. 2006;66:1294–1305. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

