IL-10-producing regulatory B10 cells inhibit intestinal injury in a mouse model
- PMID: 21281806
- PMCID: PMC3069829
- DOI: 10.1016/j.ajpath.2010.10.022
IL-10-producing regulatory B10 cells inhibit intestinal injury in a mouse model
Abstract
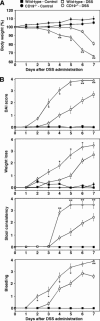
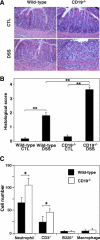
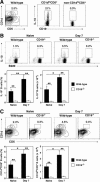
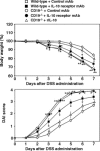
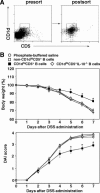
B cells mediate multiple functions that influence immune and inflammatory responses. In mice, the addition of dextran sulfate sodium (DSS) to drinking water leads to immediate intestinal injury. Dextran sulfate sodium-induced intestinal injury serves as an experimental animal model for human ulcerative colitis. The contribution of B cells to DSS-induced intestinal injury is unclear. In this study, we show that DSS-induced intestinal injury was more severe in CD19-deficient (CD19(-/-)) mice than in wild-type mice. These inflammatory responses were negatively regulated by a unique IL-10-producing CD1d(hi)CD5(+) regulatory B cell subset (B10 cells) that was absent in CD19(-/-) mice and represented only 1% to 2% of splenic B220(+) cells in wild-type mice. Remarkably, adoptive transfer of these B10 cells from wild-type mice reduced inflammation in CD19(-/-) mice in an IL-10-dependent manner. These results demonstrate that IL-10 production from regulatory B10 cells regulates DSS-induced intestinal injury. These findings may provide new insights and therapeutic approaches for treating ulcerative colitis.
Copyright © 2011 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Fiocchi C. Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology. 1998;115:182–205. - PubMed
-
- Hibi T., Ogata H. Novel pathophysiological concepts of inflammatory bowel disease. J Gastroenterol. 2006;41:10–16. - PubMed
-
- Okayasu I., Hatakeyama S., Yamada M., Ohkusa T., Inagaki Y., Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98:694–702. - PubMed
-
- Cooper H.S., Murthy S.N., Shah R.S., Sedergran D.J. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69:238–249. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

