Tenascin C induces epithelial-mesenchymal transition-like change accompanied by SRC activation and focal adhesion kinase phosphorylation in human breast cancer cells
- PMID: 21281808
- PMCID: PMC3069868
- DOI: 10.1016/j.ajpath.2010.10.015
Tenascin C induces epithelial-mesenchymal transition-like change accompanied by SRC activation and focal adhesion kinase phosphorylation in human breast cancer cells
Abstract
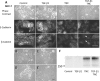
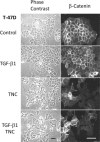
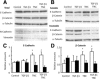
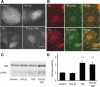
Tenascin C (TNC) is an extracellular matrix glycoprotein up-regulated in solid tumors. Higher TNC expression is shown in invading fronts of breast cancer, which correlates with poorer patient outcome. We examined whether TNC induces epithelial-mesenchymal transition (EMT) in breast cancer. Immunohistochemical analysis of invasive ductal carcinomas showed that TNC deposition was frequent in stroma with scattered cancer cells in peripheral margins of tumors. The addition of TNC to the medium of the MCF-7 breast cancer cells caused EMT-like change and delocalization of E-cadherin and β-catenin from cell-cell contact. Although amounts of E-cadherin and β-catenin were not changed after EMT in total lysates, they were increased in the Triton X-100-soluble fractions, indicating movement from the membrane into the cytosol. In wound healing assay, cells were scattered from wound edges and showed faster migration after TNC treatment. The EMT phenotype was correlated with SRC activation through phosphorylation at Y418 and phosphorylation of focal adhesion kinase (FAK) at Y861 and Y925 of SRC substrate sites. These phosphorylated proteins colocalized with αv integrin-positive adhesion plaques. A neutralizing antibody against αv or a SRC kinase inhibitor blocked EMT. TNC could induce EMT-like change showing loss of intercellular adhesion and enhanced migration in breast cancer cells, associated with FAK phosphorylation by SRC; this may be responsible for the observed promotion of TNC in breast cancer invasion.
Copyright © 2011 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Savagner P. Leaving the neighborhood: molecular mechanisms involved during epithelial-mesenchymal transition. Bioessays. 2001;23:912–923. - PubMed
-
- Thiery J.P., Acloque H., Huang R.Y., Nieto M.A. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. - PubMed
-
- Baum B., Settleman J., Quinlan M.P. Transitions between epithelial and mesenchymal states in development and disease. Semin Cell Dev Biol. 2008;19:294–308. - PubMed
-
- Yang J., Weinberg R.A. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell. 2008;14:818–829. - PubMed
-
- Thompson E.W., Newgreen D.F., Tarin D. Carcinoma invasion and metastasis: a role for epithelial-mesenchymal transition? Cancer Res. 2005;65:5991–5995. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

