Inhibition of activin receptor type IIB increases strength and lifespan in myotubularin-deficient mice
- PMID: 21281811
- PMCID: PMC3069865
- DOI: 10.1016/j.ajpath.2010.10.035
Inhibition of activin receptor type IIB increases strength and lifespan in myotubularin-deficient mice
Erratum in
- Am J Pathol. 2011 Mar;178(3):1406
Abstract
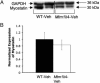
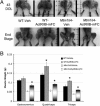
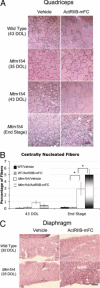
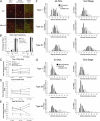
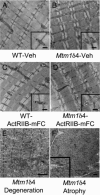
X-linked myotubular myopathy (XLMTM) is a congenital disorder caused by deficiency of the lipid phosphatase, myotubularin. Patients with XLMTM often have severe perinatal weakness that requires mechanical ventilation to prevent death from respiratory failure. Muscle biopsy specimens from patients with XLMTM exhibit small myofibers with central nuclei and central aggregations of organelles in many cells. It was postulated that therapeutically increasing muscle fiber size would cause symptomatic improvement in myotubularin deficiency. Recent studies have elucidated an important role for the activin-receptor type IIB (ActRIIB) in regulation of muscle growth and have demonstrated that ActRIIB inhibition results in significant muscle hypertrophy. To evaluate whether promoting muscle hypertrophy can attenuate symptoms resulting from myotubularin deficiency, the effect of ActRIIB-mFC treatment was determined in myotubularin-deficient (Mtm1δ4) mice. Compared with wild-type mice, untreated Mtm1δ4 mice have decreased body weight, skeletal muscle hypotrophy, and reduced survival. Treatment of Mtm1δ4 mice with ActRIIB-mFC produced a 17% extension of lifespan, with transient increases in weight, forelimb grip strength, and myofiber size. Pathologic analysis of Mtm1δ4 mice during treatment revealed that ActRIIB-mFC produced marked hypertrophy restricted to type 2b myofibers, which suggests that oxidative fibers in Mtm1δ4 animals are incapable of a hypertrophic response in this setting. These results support ActRIIB-mFC as an effective treatment for the weakness observed in myotubularin deficiency.
Copyright © 2011 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Differential muscle hypertrophy is associated with satellite cell numbers and Akt pathway activation following activin type IIB receptor inhibition in Mtm1 p.R69C mice.Am J Pathol. 2014 Jun;184(6):1831-42. doi: 10.1016/j.ajpath.2014.03.003. Epub 2014 Apr 13. Am J Pathol. 2014. PMID: 24726641 Free PMC article.
-
A soluble activin type IIB receptor improves function in a mouse model of amyotrophic lateral sclerosis.Exp Neurol. 2009 Jun;217(2):258-68. doi: 10.1016/j.expneurol.2009.02.017. Epub 2009 Mar 11. Exp Neurol. 2009. PMID: 19285073
-
Treatment with ActRIIB-mFc Produces Myofiber Growth and Improves Lifespan in the Acta1 H40Y Murine Model of Nemaline Myopathy.Am J Pathol. 2016 Jun;186(6):1568-81. doi: 10.1016/j.ajpath.2016.02.008. Epub 2016 Apr 18. Am J Pathol. 2016. PMID: 27102768 Free PMC article.
-
Interference with myostatin/ActRIIB signaling as a therapeutic strategy for Duchenne muscular dystrophy.Curr Gene Ther. 2012 Jun;12(3):245-59. doi: 10.2174/156652312800840577. Curr Gene Ther. 2012. PMID: 22554312 Review.
-
X-linked myotubular myopathy.Neuromuscul Disord. 2021 Oct;31(10):1004-1012. doi: 10.1016/j.nmd.2021.08.003. Neuromuscul Disord. 2021. PMID: 34736623 Review.
Cited by
-
Tamoxifen prolongs survival and alleviates symptoms in mice with fatal X-linked myotubular myopathy.Nat Commun. 2018 Nov 19;9(1):4848. doi: 10.1038/s41467-018-07058-4. Nat Commun. 2018. PMID: 30451843 Free PMC article.
-
Myostatin inhibitors as therapies for muscle wasting associated with cancer and other disorders.Curr Opin Support Palliat Care. 2013 Dec;7(4):352-60. doi: 10.1097/SPC.0000000000000013. Curr Opin Support Palliat Care. 2013. PMID: 24157714 Free PMC article. Review.
-
Modeling the human MTM1 p.R69C mutation in murine Mtm1 results in exon 4 skipping and a less severe myotubular myopathy phenotype.Hum Mol Genet. 2012 Feb 15;21(4):811-25. doi: 10.1093/hmg/ddr512. Epub 2011 Nov 7. Hum Mol Genet. 2012. PMID: 22068590 Free PMC article.
-
Myostatin: a Circulating Biomarker Correlating with Disease in Myotubular Myopathy Mice and Patients.Mol Ther Methods Clin Dev. 2020 May 4;17:1178-1189. doi: 10.1016/j.omtm.2020.04.022. eCollection 2020 Jun 12. Mol Ther Methods Clin Dev. 2020. PMID: 32514412 Free PMC article.
-
Pharmacology of manipulating lean body mass.Clin Exp Pharmacol Physiol. 2015 Jan;42(1):1-13. doi: 10.1111/1440-1681.12320. Clin Exp Pharmacol Physiol. 2015. PMID: 25311629 Free PMC article. Review.
References
-
- Heckmatt J.Z., Sewry C.A., Hodes D., Dubowitz V. Congenital centronuclear (myotubular) myopathy: a clinical, pathological and genetic study in eight children. Brain. 1985;108(Pt 4):941–964. - PubMed
-
- Buj-Bello A., Fougerousse F., Schwab Y., Messaddeq N., Spehner D., Pierson C.R., Durand M., Kretz C., Danos O., Douar A.M., Beggs A.H., Schultz P., Montus M., Denefle P., Mandel J.L. AAV-mediated intramuscular delivery of myotubularin corrects the myotubular myopathy phenotype in targeted murine muscle and suggests a function in plasma membrane homeostasis. Hum Mol Genet. 2008;17:2132–2143. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

