Ophthalmic pterygium: a stem cell disorder with premalignant features
- PMID: 21281814
- PMCID: PMC3069871
- DOI: 10.1016/j.ajpath.2010.10.037
Ophthalmic pterygium: a stem cell disorder with premalignant features
Abstract
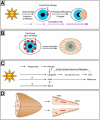
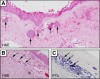
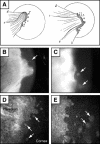
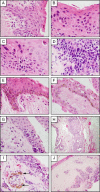
Pterygia are common ocular surface lesions thought to originate from limbal stem cells altered by chronic UV exposure. Traditionally regarded as a degenerative condition, pterygia also display tumor-like features, such as a propensity to invade normal tissue and high recurrence rates following resection, and may coexist with secondary premalignant lesions. This study was initiated to determine the rate of concurrent ocular surface diseases in patients with pterygia recruited from the practice of a single surgeon operating in a Sydney metropolitan hospital. One hundred pterygium specimens were histopathologically reviewed and selected cases were immunohistochemically assessed to confirm diagnosis. Along with previously documented typical features including epithelial proliferation, goblet cell hyperplasia, angiogenesis, inflammation, elastosis, stromal plaques, and Bowman's membrane dissolution, we identified five cases of ocular surface squamous neoplasia, six cases of primary acquired melanosis, two compound nevi (one suspect invasive melanoma), and one dermoid-like lesion. In 18 specimens, clusters of basal epithelial cells that coexpressed cytokeratin-15/-19 and p63-α were identified at the head of the pterygium, coinciding with clinical observation of Fuchs' flecks. Our data show that significant preneoplastic lesions may be associated with pterygium and that all excised pterygia should undergo histological examination. The presence of p63-α-positive epithelial cell clusters supports the hypothesis that pterygia develop from limbal epithelial progenitors.
Copyright © 2011 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures


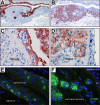
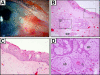
References
-
- Duke-Elder S., editor. Diseases of the Outer Eye Part 1: System of Ophthalmology 8. Kimpton; London: 1965. pp. 569–585.
-
- Di Girolamo N., Chui J., Coroneo M.T., Wakefield D. Pathogenesis of pterygia: role of cytokines, growth factors, and matrix metalloproteinases. Prog Retin Eye Res. 2004;23:195–228. - PubMed
-
- Di Girolamo N., McCluskey P., Lloyd A., Coroneo M.T., Wakefield D. Expression of MMPs and TIMPs in human pterygia and cultured pterygium epithelial cells. Invest Ophthalmol Vis Sci. 2000;41:671–679. - PubMed
-
- Di Girolamo N., Coroneo M.T., Wakefield D. Active matrilysin (MMP-7) in human pterygia: potential role in angiogenesis. Invest Ophthalmol Vis Sci. 2001;42:1963–1968. - PubMed
-
- Di Girolamo N., Kumar R.K., Coroneo M.T., Wakefield D. UVB-mediated induction of interleukin-6 and -8 in pterygia and cultured human pterygium epithelial cells. Invest Ophthalmol Vis Sci. 2002;43:3430–3437. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

