Isolation and characterization of progenitor-like cells from human renal proximal tubules
- PMID: 21281815
- PMCID: PMC3070548
- DOI: 10.1016/j.ajpath.2010.10.026
Isolation and characterization of progenitor-like cells from human renal proximal tubules
Abstract
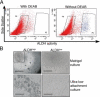
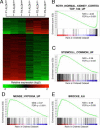
The tubules of the kidney display a remarkable capacity for self-renewal on damage. Whether this regeneration is mediated by dedifferentiating surviving cells or, as recently suggested, by stem cells has not been unequivocally settled. Herein, we demonstrate that aldehyde dehydrogenase (ALDH) activity may be used for isolation of cells with progenitor characteristics from adult human renal cortical tissue. Gene expression profiling of the isolated ALDH(high) and ALDH(low) cell fractions followed by immunohistochemical interrogation of renal tissues enabled us to delineate a tentative progenitor cell population scattered through the proximal tubules (PTs). These cells expressed CD24 and CD133, previously described markers for renal progenitors of Bowman's capsule. Furthermore, we show that the PT cells, and the glomerular progenitors, are positive for KRT7, KRT19, BCL2, and vimentin. In addition, tubular epithelium regenerating on acute tubular necrosis displayed long stretches of CD133(+)/VIM(+) cells, further substantiating that these cells may represent a progenitor cell population. Furthermore, a potential association of these progenitor cells with papillary renal cell carcinoma was discovered. Taken together, our data demonstrate the presence of a previously unappreciated subset of the PT cells that may be endowed with a more robust phenotype, allowing increased resistance to acute renal injury, enabling rapid repopulation of the tubules.
Copyright © 2011 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures




Comment in
-
Family portrait: renal progenitor of Bowman's capsule and its tubular brothers.Am J Pathol. 2011 Feb;178(2):490-3. doi: 10.1016/j.ajpath.2010.11.044. Am J Pathol. 2011. PMID: 21281781 Free PMC article. No abstract available.
References
-
- Ronconi E., Sagrinati C., Angelotti M.L., Lazzeri E., Mazzinghi B., Ballerini L., Parente E., Becherucci F., Gacci M., Carini M., Maggi E., Serio M., Vannelli G.B., Lasagni L., Romagnani S., Romagnani P. Regeneration of glomerular podocytes by human renal progenitors. J Am Soc Nephrol. 2009;20:322–332. - PMC - PubMed
-
- Sagrinati C., Netti G.S., Mazzinghi B., Lazzeri E., Liotta F., Frosali F., Ronconi E., Meini C., Gacci M., Squecco R., Carini M., Gesualdo L., Francini F., Maggi E., Annunziato F., Lasagni L., Serio M., Romagnani S., Romagnani P. Isolation and characterization of multipotent progenitor cells from the Bowman's capsule of adult human kidneys. J Am Soc Nephrol. 2006;17:2443–2456. - PubMed
-
- Vogetseder A., Palan T., Bacic D., Kaissling B., Le Hir M. Proximal tubular epithelial cells are generated by division of differentiated cells in the healthy kidney. Am J Physiol Cell Physiol. 2007;292:C807–C813. - PubMed
-
- Vogetseder A., Picard N., Gaspert A., Walch M., Kaissling B., Le Hir M. Proliferation capacity of the renal proximal tubule involves the bulk of differentiated epithelial cells. Am J Physiol Cell Physiol. 2008;294:C22–C28. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

