Inflammation alters trafficking of extrasynaptic AMPA receptors in tonically firing lamina II neurons of the rat spinal dorsal horn
- PMID: 21282008
- PMCID: PMC3079375
- DOI: 10.1016/j.pain.2011.01.016
Inflammation alters trafficking of extrasynaptic AMPA receptors in tonically firing lamina II neurons of the rat spinal dorsal horn
Abstract
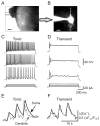
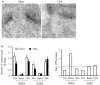
Peripheral inflammation alters AMPA receptor (AMPAR) subunit trafficking and increases AMPAR Ca(2+) permeability at synapses of spinal dorsal horn neurons. However, it is unclear whether AMPAR trafficking at extrasynaptic sites of these neurons also changes under persistent inflammatory pain conditions. Using patch-clamp recording combined with Ca(2+) imaging and cobalt staining, we found that, under normal conditions, an extrasynaptic pool of AMPARs in rat substantia gelatinosa (SG) neurons of spinal dorsal horn predominantly consists of GluR2-containing Ca(2+)-impermeable receptors. Maintenance of complete Freund's adjuvant (CFA)-induced inflammation was associated with a marked enhancement of AMPA-induced currents and [Ca(2+)](i) transients in SG neurons, while, as we previously showed, the amplitude of synaptically evoked AMPAR-mediated currents was not changed 24 h after CFA. These findings indicate that extrasynaptic AMPARs are upregulated and their Ca(2+) permeability increases dramatically. This increase occurred in SG neurons characterized by intrinsic tonic firing properties, but not in those exhibited strong adaptation. This increase was also accompanied by an inward rectification of AMPA-induced currents and enhancement of sensitivity to a highly selective Ca(2+)-permeable AMPAR blocker, IEM-1460. Electron microcopy and biochemical assays additionally showed an increase in the amount of GluR1 at extrasynaptic membranes in dorsal horn neurons 24h post-CFA. Taken together, our findings indicate that CFA-induced inflammation increases functional expression and proportion of extrasynaptic GluR1-containing Ca(2+)-permeable AMPARs in tonically firing excitatory dorsal horn neurons, suggesting that the altered extrasynaptic AMPAR trafficking might participate in the maintenance of persistent inflammatory pain.
Copyright © 2011 International Association for the Study of Pain. All rights reserved.
Conflict of interest statement
The authors declare no conflict of interests.
Figures





References
-
- Albuquerque C, Lee CJ, Jackson AC, MacDermott AB. Subpopulations of GABAergic and non-GABAergic rat dorsal horn neurons express Ca2+-permeable AMPA receptors. Eur J Neurosci. 1999;11:2758–2766. - PubMed
-
- Allan SM, Rothwell NJ. Cytokines and acute neurodegeneration. Nat Rev Neurosci. 2001;2:734–744. - PubMed
-
- Atianjoh FE, Yaster M, Zhao X, Takamiya K, Xia J, Gauda EB, Huganir RL, Tao YX. Spinal cord protein interacting with C kinase 1 is required for the maintenance of complete Freund’s adjuvant-induced inflammatory pain but not for incision-induced post-operative pain. Pain. 2010;151:226–234. - PMC - PubMed
-
- Balasubramanyan S, Stemkowski PL, Stebbing MJ, Smith PA. Sciatic chronic constriction injury produces cell-type-specific changes in the electrophysiological properties of rat substantia gelatinosa neurons. J Neurophysiol. 2006;96:579–590. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

