Tyrosine latching of a regulatory gate affords allosteric control of aromatic amino acid biosynthesis
- PMID: 21282100
- PMCID: PMC3060475
- DOI: 10.1074/jbc.M110.209924
Tyrosine latching of a regulatory gate affords allosteric control of aromatic amino acid biosynthesis
Abstract
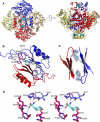
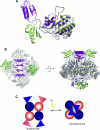
The first step of the shikimate pathway for aromatic amino acid biosynthesis is catalyzed by 3-deoxy-D-arabino-heptulosonate 7-phosphate synthase (DAH7PS). Thermotoga maritima DAH7PS (TmaDAH7PS) is tetrameric, with monomer units comprised of a core catalytic (β/α)(8) barrel and an N-terminal domain. This enzyme is inhibited strongly by tyrosine and to a lesser extent by the presence of phenylalanine. A truncated mutant of TmaDAH7PS lacking the N-terminal domain was catalytically more active and completely insensitive to tyrosine and phenylalanine, consistent with a role for this domain in allosteric inhibition. The structure of this protein was determined to 2.0 Å. In contrast to the wild-type enzyme, this enzyme is dimeric. Wild-type TmaDAH7PS was co-crystallized with tyrosine, and the structure of this complex was determined to a resolution of 2.35 Å. Tyrosine was found to bind at the interface between two regulatory N-terminal domains, formed from diagonally located monomers of the tetramer, revealing a major reorganization of the regulatory domain with respect to the barrel relative to unliganded enzyme. This significant conformational rearrangement observed in the crystal structures was also clearly evident from small angle X-ray scattering measurements recorded in the presence and absence of tyrosine. The closed conformation adopted by the protein on tyrosine binding impedes substrate entry into the neighboring barrel, revealing an unusual tyrosine-controlled gating mechanism for allosteric control of this enzyme.
Figures




References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

