A genome-wide chromatin-associated nuclear peroxiredoxin from the malaria parasite Plasmodium falciparum
- PMID: 21282103
- PMCID: PMC3064226
- DOI: 10.1074/jbc.M110.198499
A genome-wide chromatin-associated nuclear peroxiredoxin from the malaria parasite Plasmodium falciparum
Abstract
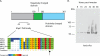
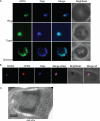
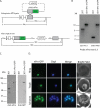
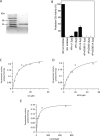
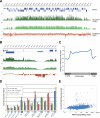
Malaria parasites are subjected to high levels of oxidative stress during their development inside erythrocytes and the ability of the parasite to defend itself against this assault is critical to its survival. Therefore, Plasmodium possesses an effective antioxidant defense system that could potentially be used as a target for the development of inhibitor-based therapy. We have identified an unusual peroxiredoxin protein that localizes to the nucleus of Plasmodium falciparum and have renamed it PfnPrx (PF10_0268, earlier called MCP1). Our work reveals that PfnPrx has a broad specificity of substrate being able to utilize thioredoxin and glutaredoxin as reductants and having the ability to reduce simple and complex peroxides. Intriguingly, chromatin immunoprecipitation followed by deep sequencing reveals that the enzyme associates with chromatin in a genome-wide manner with a slight enrichment in coding regions. Our results represent the first description of a dedicated chromatin-associated peroxiredoxin and potentially represent an ingenious way by which the parasite can survive the highly oxidative environment within its human host.
Figures
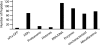
Similar articles
-
Thioredoxin and glutathione systems in Plasmodium falciparum.Int J Med Microbiol. 2012 Oct;302(4-5):187-94. doi: 10.1016/j.ijmm.2012.07.007. Epub 2012 Aug 29. Int J Med Microbiol. 2012. PMID: 22939033 Review.
-
Redox interactome in malaria parasite Plasmodium falciparum.Parasitol Res. 2021 Feb;120(2):423-434. doi: 10.1007/s00436-021-07051-9. Epub 2021 Jan 18. Parasitol Res. 2021. PMID: 33459846 Review.
-
Thioredoxin networks in the malarial parasite Plasmodium falciparum.Antioxid Redox Signal. 2006 Jul-Aug;8(7-8):1227-39. doi: 10.1089/ars.2006.8.1227. Antioxid Redox Signal. 2006. PMID: 16910770 Review.
-
Redox and antioxidant systems of the malaria parasite Plasmodium falciparum.Mol Microbiol. 2004 Sep;53(5):1291-305. doi: 10.1111/j.1365-2958.2004.04257.x. Mol Microbiol. 2004. PMID: 15387810 Review.
-
Plasmodium falciparum antioxidant protein as a model enzyme for a special class of glutaredoxin/glutathione-dependent peroxiredoxins.Biochim Biophys Acta. 2013 Aug;1830(8):4073-90. doi: 10.1016/j.bbagen.2013.04.020. Epub 2013 Apr 24. Biochim Biophys Acta. 2013. PMID: 23624334
Cited by
-
Absence of Peroxiredoxin 6 Amplifies the Effect of Oxidant Stress on Mobility and SCSA/CMA3 Defined Chromatin Quality and Impairs Fertilizing Ability of Mouse Spermatozoa.Biol Reprod. 2016 Mar;94(3):68. doi: 10.1095/biolreprod.115.137646. Epub 2016 Jan 20. Biol Reprod. 2016. PMID: 26792942 Free PMC article.
-
Identification and functional analysis of a novel mitochondria-localized 2-Cys peroxiredoxin, BbTPx-2, from Babesia bovis.Parasitol Res. 2016 Aug;115(8):3139-45. doi: 10.1007/s00436-016-5071-9. Epub 2016 Apr 19. Parasitol Res. 2016. PMID: 27095567
-
Plasmodium knowlesi thioredoxin peroxidase 1 binds to nucleic acids and has RNA chaperone activity.Parasitol Res. 2014 Nov;113(11):3957-62. doi: 10.1007/s00436-014-4060-0. Epub 2014 Aug 5. Parasitol Res. 2014. PMID: 25092384
-
Chemo-proteomics in antimalarial target identification and engagement.Med Res Rev. 2023 Nov;43(6):2303-2351. doi: 10.1002/med.21975. Epub 2023 May 26. Med Res Rev. 2023. PMID: 37232495 Free PMC article. Review.
-
Advancing age increases sperm chromatin damage and impairs fertility in peroxiredoxin 6 null mice.Redox Biol. 2015 Aug;5:15-23. doi: 10.1016/j.redox.2015.02.004. Epub 2015 Feb 25. Redox Biol. 2015. PMID: 25796034 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

