N-terminal glutamate to pyroglutamate conversion in vivo for human IgG2 antibodies
- PMID: 21282104
- PMCID: PMC3064176
- DOI: 10.1074/jbc.M110.185041
N-terminal glutamate to pyroglutamate conversion in vivo for human IgG2 antibodies
Abstract
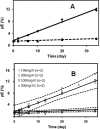
Therapeutic proteins contain a large number of post-translational modifications, some of which could potentially impact their safety or efficacy. In one of these changes, pyroglutamate can form on the N terminus of the polypeptide chain. Both glutamine and glutamate at the N termini of recombinant monoclonal antibodies can cyclize spontaneously to pyroglutamate (pE) in vitro. Glutamate conversion to pyroglutamate occurs more slowly than from glutamine but has been observed under near physiological conditions. Here we investigated to what extent human IgG2 N-terminal glutamate converts to pE in vivo. Pyroglutamate levels increased over time after injection into humans, with the rate of formation differing between polypeptide chains. These changes were replicated for the same antibodies in vitro under physiological pH and temperature conditions, indicating that the changes observed in vivo were due to chemical conversion not differential clearance. Differences in the conversion rates between the light chain and heavy chain on an antibody were eliminated by denaturing the protein, revealing that structural elements affect pE formation rates. By enzymatically releasing pE from endogenous antibodies isolated from human serum, we could estimate the naturally occurring levels of this post-translational modification. Together, these techniques and results can be used to predict the exposure of pE for therapeutic antibodies and to guide criticality assessments for this attribute.
Figures




References
-
- Blomback B. (1967) in Methods in Enzymology (Hirs C. ed) Vol. 11, pp. 389–411, Academic Press, New York
-
- Schilling S., Wasternack C., Demuth H. U. (2008) Biol. Chem. 389, 983–991 - PubMed
-
- Dayhoff M. O. (ed) (1972) Atlas of Protein Sequence and Structure, National Biomedical Research Foundation, Silver Spring, MD
-
- Ouellette D., Alessandri L., Chin A., Grinnell C., Tarcsa E., Radziejewski C., Correia I. (2010) Anal. Biochem. 397, 37–47 - PubMed
-
- Dick L. W., Jr., Kim C., Qiu D., Cheng K. C. (2007) Biotechnol. Bioeng. 97, 544–553 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

