Bone marrow CD169+ macrophages promote the retention of hematopoietic stem and progenitor cells in the mesenchymal stem cell niche
- PMID: 21282381
- PMCID: PMC3039855
- DOI: 10.1084/jem.20101688
Bone marrow CD169+ macrophages promote the retention of hematopoietic stem and progenitor cells in the mesenchymal stem cell niche
Abstract
Hematopoietic stem cells (HSCs) reside in specialized bone marrow (BM) niches regulated by the sympathetic nervous system (SNS). Here, we have examined whether mononuclear phagocytes modulate the HSC niche. We defined three populations of BM mononuclear phagocytes that include Gr-1(hi) monocytes (MOs), Gr-1(lo) MOs, and macrophages (MΦ) based on differential expression of Gr-1, CD115, F4/80, and CD169. Using MO and MΦ conditional depletion models, we found that reductions in BM mononuclear phagocytes led to reduced BM CXCL12 levels, the selective down-regulation of HSC retention genes in Nestin(+) niche cells, and egress of HSCs/progenitors to the bloodstream. Furthermore, specific depletion of CD169(+) MΦ, which spares BM MOs, was sufficient to induce HSC/progenitor egress. MΦ depletion also enhanced mobilization induced by a CXCR4 antagonist or granulocyte colony-stimulating factor. These results highlight two antagonistic, tightly balanced pathways that regulate maintenance of HSCs/progenitors in the niche during homeostasis, in which MΦ cross talk with the Nestin(+) niche cell promotes retention, and in contrast, SNS signals enhance egress. Thus, strategies that target BM MΦ hold the potential to augment stem cell yields in patients that mobilize HSCs/progenitors poorly.
Figures
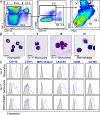

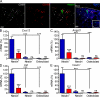

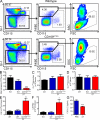
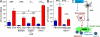

References
-
- Berg D.J., Davidson N., Kuhn R., Muller W., Menon S., Holland G., Thompson-Snipes L., Leach M.W., Rennick D. 1996. Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4(+) TH1-like responses. J. Clin. Invest. 98:1010–1020 10.1172/JCI118861 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30CA013330/CA/NCI NIH HHS/United States
- R01 HL069438/HL/NHLBI NIH HHS/United States
- R01CA112100/CA/NCI NIH HHS/United States
- 1F30HL099028-01/HL/NHLBI NIH HHS/United States
- R01 HL097819/HL/NHLBI NIH HHS/United States
- R01 HL116340/HL/NHLBI NIH HHS/United States
- R01 CA112100/CA/NCI NIH HHS/United States
- R01HL097819/HL/NHLBI NIH HHS/United States
- F30 HL099028/HL/NHLBI NIH HHS/United States
- R01DK056638/DK/NIDDK NIH HHS/United States
- P30 CA013330/CA/NCI NIH HHS/United States
- R01 DK056638/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

