CD4+ T cells are not essential for control of early acute Cryptosporidium parvum infection in neonatal mice
- PMID: 21282414
- PMCID: PMC3067537
- DOI: 10.1128/IAI.00922-10
CD4+ T cells are not essential for control of early acute Cryptosporidium parvum infection in neonatal mice
Abstract
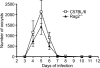
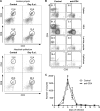
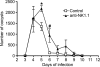
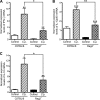
Cryptosporidiosis is an important diarrheal disease of humans and neonatal livestock caused by Cryptosporidium spp. that infect epithelial cells. Recovery from Cryptosporidium parvum infection in adult hosts involves CD4(+) T cells with a strong Th1 component, but mechanisms of immunity in neonates are not well characterized. In the present investigation with newborn mice, similar acute patterns of infection were obtained in C57BL/6 wild-type (WT) and T and B cell-deficient Rag2(-/-) mice. In comparison with uninfected controls, the proportion of intestinal CD4(+) or CD8(+) T cells did not increase in infected WT mice during recovery from infection. Furthermore, infection in neonatal WT mice depleted of CD4(+) T cells was not exacerbated. Ten weeks after WT and Rag2(-/-) mice had been infected as neonates, no patent infections could be detected. Treatment at this stage with the immunosuppressive drug dexamethasone produced patent infections in Rag2(-/-) mice but not WT mice. Expression of inflammatory markers, including gamma interferon (IFN-γ) and interleukin-12p40 (IL-12p40), was higher in neonatal WT mice than in Rag2(-/-) mice around the peak of infection, but IL-10 expression was also higher in WT mice. These results suggest that although CD4(+) T cells may be important for elimination of C. parvum, these cells are dispensable for controlling the early acute phase of infection in neonates.
Figures

Similar articles
-
IL-4 protects adult C57BL/6 mice from prolonged Cryptosporidium parvum infection: analysis of CD4+alpha beta+IFN-gamma+ and CD4+alpha beta+IL-4+ lymphocytes in gut-associated lymphoid tissue during resolution of infection.J Immunol. 1998 Aug 15;161(4):1891-900. J Immunol. 1998. PMID: 9712058
-
Dynamics of gut mucosal and systemic Th1/Th2 cytokine responses in interferon-gamma and interleukin-12p40 knock out mice during primary and challenge Cryptosporidium parvum infection.Immunobiology. 2009;214(6):454-66. doi: 10.1016/j.imbio.2008.11.015. Epub 2009 Jan 19. Immunobiology. 2009. PMID: 19155092
-
Cryptosporidium parvum-specific mucosal immune response in C57BL/6 neonatal and gamma interferon-deficient mice: role of tumor necrosis factor alpha in protection.Infect Immun. 2001 Mar;69(3):1635-42. doi: 10.1128/IAI.69.3.1635-1642.2001. Infect Immun. 2001. PMID: 11179338 Free PMC article.
-
Innate immune responses against Cryptosporidium parvum infection.Parasite Immunol. 2013 Feb;35(2):55-64. doi: 10.1111/pim.12020. Parasite Immunol. 2013. PMID: 23173616 Review.
-
Host cell-mediated responses to infection with Cryptosporidium.Parasite Immunol. 2000 Dec;22(12):597-604. doi: 10.1046/j.1365-3024.2000.00343.x. Parasite Immunol. 2000. PMID: 11123751 Review.
Cited by
-
Systemic and Mucosal Immune Responses to Cryptosporidium-Vaccine Development.Curr Trop Med Rep. 2015 Sep 1;2(3):171-180. doi: 10.1007/s40475-015-0054-y. Curr Trop Med Rep. 2015. PMID: 26279971 Free PMC article.
-
Non-coding RNAs in epithelial immunity to Cryptosporidium infection.Parasitology. 2014 Sep;141(10):1233-43. doi: 10.1017/S0031182014000614. Epub 2014 May 14. Parasitology. 2014. PMID: 24828969 Free PMC article. Review.
-
The early intestinal immune response in experimental neonatal ovine cryptosporidiosis is characterized by an increased frequency of perforin expressing NCR1(+) NK cells and by NCR1(-) CD8(+) cell recruitment.Vet Res. 2015 Mar 11;46:28. doi: 10.1186/s13567-014-0136-1. Vet Res. 2015. PMID: 25890354 Free PMC article.
-
Cellular immune response and scanning electron microscopy in the evaluation of Moringa leaves aqueous extract effect on Cryptosporidium parvum in buffalo intestinal tissue explants.J Parasit Dis. 2019 Sep;43(3):393-401. doi: 10.1007/s12639-019-01103-9. Epub 2019 Mar 18. J Parasit Dis. 2019. PMID: 31406404 Free PMC article.
-
Innate Lymphoid Cells in Protection, Pathology, and Adaptive Immunity During Apicomplexan Infection.Front Immunol. 2019 Feb 28;10:196. doi: 10.3389/fimmu.2019.00196. eCollection 2019. Front Immunol. 2019. PMID: 30873151 Free PMC article. Review.
References
-
- Adkins, B., C. Leclerc, and S. Marshall-Clarke. 2004. Neonatal adaptive immunity comes of age. Nat. Rev. Immunol. 4:553-564. - PubMed
-
- Barrier, M., et al. 2006. Oral and intraperitoneal administration of phosphorothioate oligodeoxynucleotides leads to control of Cryptosporidium parvum infection in neonatal mice. J. Infect. Dis. 193:1400-1407. - PubMed
-
- Campbell, L. D., J. N. Stewart, and J. R. Mead. 2002. Susceptibility to Cryptosporidium parvum infections in cytokine- and chemokine-receptor knockout mice. J. Parasitol. 88:1014-1016. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

