In vivo efficacy of the novel aminoglycoside ACHN-490 in murine infection models
- PMID: 21282439
- PMCID: PMC3067188
- DOI: 10.1128/AAC.00862-10
In vivo efficacy of the novel aminoglycoside ACHN-490 in murine infection models
Abstract
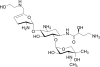
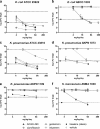
Aminoglycosides are broad-spectrum antibiotics with particular clinical utility against life-threatening infections. As resistance to antibiotics, including aminoglycosides, continues to grow, there is a need for new and effective antimicrobial agents. ACHN-490 is a novel aminoglycoside in clinical development with activity against multidrug-resistant Gram-negative and select Gram-positive pathogens. Here we assess the in vivo efficacy of ACHN-490 against a variety of common pathogens in two murine models: the septicemia and neutropenic thigh models. When its activity against a gentamicin-susceptible strain of Escherichia coli was tested in the septicemia model, ACHN-490 improved 7-day survival with a dose-response profile similar to that of gentamicin, with 100% survival seen at doses of 1.6 mg/kg of body weight and above. In animals infected with a gentamicin-susceptible strain of Pseudomonas aeruginosa, treatment with either ACHN-490 or gentamicin led to 100% survival at doses of 16 mg/kg and above in the septicemia model. ACHN-490 was also effective in the neutropenic thigh model, reducing multidrug-resistant Enterobacteriaceae family and methicillin-resistant Staphylococcus aureus strains, as well as broadly susceptible strains, to static levels with dose-dependent activity. Against gentamicin-sensitive Enterobacteriaceae and methicillin-resistant S. aureus, the efficacy of ACHN-490 was comparable to that of gentamicin. However, gentamicin-resistant Enterobacteriaceae strains and those harboring the Klebsiella pneumoniae carbapenemase responded to ACHN-490 but not gentamicin, with static doses ranging from 12 mg/kg to 64 mg/kg for ACHN-490. These results suggest that ACHN-490 has the potential to become a clinically useful agent against drug-resistant pathogens, including Enterobacteriaceae, P. aeruginosa, and methicillin-resistant S. aureus, and support further development of this promising novel aminoglycoside.
Figures


References
-
- Alvarez-Lerma, F. 1996. Modification of empiric antibiotic treatment in patients with pneumonia acquired in the intensive care unit. ICU-Acquired Pneumonia Study Group. Intensive Care Med. 22:387-394. - PubMed
-
- Black, J., B. Calesnick, D. Williams, and M. J. Weinstein. 1963. Pharmacology of gentamicin, a new broad-spectrum antibiotic, p. 138-147. Antimicrob. Agents Chemother. 1962. - PubMed
-
- Bratu, S., et al. 2005. Rapid spread of carbapenem-resistant Klebsiella pneumoniae in New York City: a new threat to our antibiotic armamentarium. Arch. Intern. Med. 165:1430-1435. - PubMed
-
- Clinical and Laboratory Standards Institute. 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 7th ed. Clinical and Laboratory Standards Institute document M7-A7. Clinical and Laboratory Standards Institute, Wayne, PA.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

