Role of VltAB, an ABC transporter complex, in viologen tolerance in Streptococcus mutans
- PMID: 21282456
- PMCID: PMC3067168
- DOI: 10.1128/AAC.01094-10
Role of VltAB, an ABC transporter complex, in viologen tolerance in Streptococcus mutans
Abstract
Streptococcus mutans, a Gram-positive organism, is the primary causative agent in the formation of dental caries in humans. To persist in the oral cavity, S. mutans must be able to tolerate rapid environmental fluctuations and exposure to various toxic chemicals. However, the mechanisms underlying the ability of this cariogenic pathogen to survive and proliferate under harsh environmental conditions remain largely unknown. Here, we wanted to understand the mechanisms by which S. mutans withstands exposure to methyl viologen (MV), a quaternary ammonium compound (QAC) that generates superoxide radicals in the cell. To elucidate the essential genes for MV tolerance, screening of ∼3,500 mutants generated by ISS1 mutagenesis, revealed 15 MV-sensitive mutants. Among them, five and four independent insertions had occurred in SMU.905 and SMU.906 genes, respectively. These two genes are appeared to be organized in an operon and encode a putative ABC transporter complex; we designated the genes as vltA and vltB, for viologen transporter. To verify our results, vltA was deleted by using an antibiotic resistance marker; the mutant was just as sensitive to MV as the ISS1 insertion mutants. Furthermore, vltA and vltB mutants were also sensitive to other viologen compounds such as benzyl and ethyl viologens. Complementation assays were also carried out to confirm the role of VltA and VltB in viologen tolerance. Sensitivity to various drugs, including a wide range of QACs, was evaluated. It appears that a functional VltA is also required for full resistance toward acriflavin, ethidium bromide, and safranin; all are well-known QACs. These results indicate that VltA/B constitute a heterodimeric multidrug efflux pump of the ABC family. BLAST-P analysis suggests that homologs of VltA/B are widely present in streptococci, enterococci, and other important Gram-positive pathogens.
Figures



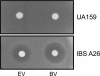
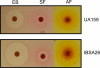

Similar articles
-
3'-Phosphoadenosine-5'-phosphate phosphatase activity is required for superoxide stress tolerance in Streptococcus mutans.J Bacteriol. 2009 Jul;191(13):4330-40. doi: 10.1128/JB.00184-09. Epub 2009 May 8. J Bacteriol. 2009. PMID: 19429620 Free PMC article.
-
Activation of the SMU.1882 transcription by CovR in Streptococcus mutans.PLoS One. 2010 Nov 22;5(11):e15528. doi: 10.1371/journal.pone.0015528. PLoS One. 2010. PMID: 21124877 Free PMC article.
-
Characterization of the Streptococcus mutans SMU.1703c-SMU.1702c Operon Reveals Its Role in Riboflavin Import and Response to Acid Stress.J Bacteriol. 2020 Dec 18;203(2):e00293-20. doi: 10.1128/JB.00293-20. Print 2020 Dec 18. J Bacteriol. 2020. PMID: 33077636 Free PMC article.
-
A novel ATP-binding cassette transporter is responsible for resistance to viologen herbicides in the cyanobacterium Synechocystis sp. PCC 6803.FEBS J. 2009 Aug;276(15):4001-11. doi: 10.1111/j.1742-4658.2009.07109.x. Epub 2009 Jul 7. FEBS J. 2009. PMID: 19594831
-
SMU.746-SMU.747, a putative membrane permease complex, is involved in aciduricity, acidogenesis, and biofilm formation in Streptococcus mutans.J Bacteriol. 2014 Jan;196(1):129-39. doi: 10.1128/JB.00960-13. Epub 2013 Oct 18. J Bacteriol. 2014. PMID: 24142257 Free PMC article.
Cited by
-
The expression of superoxide dismutase (SOD) and a putative ABC transporter permease is inversely correlated during biofilm formation in Listeria monocytogenes 4b G.PLoS One. 2012;7(10):e48467. doi: 10.1371/journal.pone.0048467. Epub 2012 Oct 31. PLoS One. 2012. PMID: 23119031 Free PMC article.
-
Characterization of a stress tolerance-defective mutant of Lactobacillus rhamnosus LRB.Mol Oral Microbiol. 2019 Aug;34(4):153-167. doi: 10.1111/omi.12262. Epub 2019 Jun 7. Mol Oral Microbiol. 2019. PMID: 31056830 Free PMC article.
-
Oxidative Stressors Modify the Response of Streptococcus mutans to Its Competence Signal Peptides.Appl Environ Microbiol. 2017 Oct 31;83(22):e01345-17. doi: 10.1128/AEM.01345-17. Print 2017 Nov 15. Appl Environ Microbiol. 2017. PMID: 28887419 Free PMC article.
-
Tooth brushing using toothpaste containing theaflavins reduces the oral pathogenic bacteria in healthy adults.3 Biotech. 2021 Mar;11(3):150. doi: 10.1007/s13205-021-02699-7. Epub 2021 Mar 2. 3 Biotech. 2021. PMID: 33747700 Free PMC article.
-
Bacterial Profile, Antimicrobial Susceptibility Pattern, and Associated Factors among Dental Caries-Suspected Patients Attending the Ayder Comprehensive Specialized Hospital and Private Dental Clinic in Mekelle, Northern Ethiopia.Biomed Res Int. 2022 Oct 17;2022:3463472. doi: 10.1155/2022/3463472. eCollection 2022. Biomed Res Int. 2022. PMID: 36299705 Free PMC article.
References
-
- Beauchamp, C., and I. Fridovich. 1971. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 44:276-287. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

