Augmented expression and activity of extracellular matrix-degrading enzymes in regions of low endothelial shear stress colocalize with coronary atheromata with thin fibrous caps in pigs
- PMID: 21282495
- PMCID: PMC3066078
- DOI: 10.1161/CIRCULATIONAHA.110.970038
Augmented expression and activity of extracellular matrix-degrading enzymes in regions of low endothelial shear stress colocalize with coronary atheromata with thin fibrous caps in pigs
Abstract
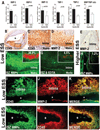
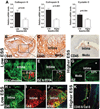
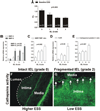
Background- The molecular mechanisms that determine the localized formation of thin-capped atheromata in the coronary arteries remain unknown. This study tested the hypothesis that low endothelial shear stress augments the expression of matrix-degrading proteases and thereby promotes the formation of thin-capped atheromata. Methods and Results- Intravascular ultrasound-based, geometrically correct 3-dimensional reconstruction of the coronary arteries of 12 swine was performed in vivo 23 weeks after initiation of diabetes mellitus and a hyperlipidemic diet. Local endothelial shear stress was calculated in plaque-free subsegments of interest (n=142) with computational fluid dynamics. At week 30, the coronary arteries (n=31) were harvested and the same subsegments were identified. The messenger RNA and protein expression and elastolytic activity of selected elastases and their endogenous inhibitors were assessed. Subsegments with low preceding endothelial shear stress at week 23 showed reduced endothelial coverage, enhanced lipid accumulation, and intense infiltration of activated inflammatory cells at week 30. These lesions showed increased expression of messenger RNAs encoding matrix metalloproteinase-2, -9, and -12, and cathepsins K and S relative to their endogenous inhibitors and increased elastolytic activity. Expression of these enzymes correlated positively with the severity of internal elastic lamina fragmentation. Thin-capped atheromata developed in regions with lower preceding endothelial shear stress and had reduced endothelial coverage, intense lipid and inflammatory cell accumulation, enhanced messenger RNA expression and elastolytic activity of MMPs and cathepsins, and severe internal elastic lamina fragmentation. Conclusions- Low endothelial shear stress induces endothelial discontinuity and accumulation of activated inflammatory cells, thereby augmenting the expression and activity of elastases in the intima and shifting the balance with their inhibitors toward matrix breakdown. Our results provide new insight into the mechanisms of regional formation of plaques with thin fibrous caps.
Figures

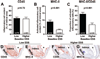

References
-
- Chatzizisis YS, Coskun AU, Jonas M, Edelman ER, Feldman CL, Stone PH. Role of endothelial shear stress in the natural history of coronary atherosclerosis and vascular remodeling: molecular, cellular and vascular behavior. J Am Coll Cardiol. 2007;49:2379–2393. - PubMed
-
- Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2000;20:1262–1275. - PubMed
-
- Chatzizisis YS, Jonas M, Coskun AU, Beigel R, Stone BV, Maynard C, Gerrity R, Daley W, Campbell R, Elazer ER, Feldman CL, Stone PH. Prediction of the localization of high-risk coronary atherosclerotic plaques on the basis of low endothelial shear stress: an intravascular ultrasound and histopathology natural history study. Circulation. 2008;117:993–1002. - PubMed
-
- Koskinas KC, Feldman CL, Chatzizisis YS, Coskun AU, Jonas M, Maynard C, Baker AB, Edelman ER, Stone PH. Natural history of experimental coronary atherosclerosis and vascular remodeling in relation to endothelial shear stress: a serial, in vivo intravascular ultrasound study. Circulation. 2010;121:2092–2101. - PMC - PubMed
-
- Cheng C, Tempel D, van Haperen R, van der Baan A, Grosveld F, Daemen MJ, Krams R, de Crom R. Atherosclerotic lesion size and vulnerability are determined by patterns of fluid shear stress. Circulation. 2006;113:2744–2753. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

