Cardiovascular molecular imaging: focus on clinical translation
- PMID: 21282520
- PMCID: PMC3073678
- DOI: 10.1161/CIRCULATIONAHA.109.916338
Cardiovascular molecular imaging: focus on clinical translation
Figures


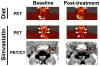





References
-
- Massoud TF, Gambhir SS. Molecular imaging in living subjects: seeing fundamental biological processes in a new light. Genes Dev. 2003;17:545–580. - PubMed
-
- Ginsburg GS, Donahue MP, Newby LK. Prospects for personalized cardiovascular medicine: the impact of genomics. J Am Coll Cardiol. 2005;46:1615–1627. - PubMed
-
- Sinusas AJ, Bengel F, Nahrendorf M, Epstein FH, Wu JC, Villanueva FS, Fayad ZA, Gropler RJ. Multimodality cardiovascular molecular imaging, part I. Circ Cardiovasc Imaging. 2008;1:244–256. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

