First-in-man study of CPX-351: a liposomal carrier containing cytarabine and daunorubicin in a fixed 5:1 molar ratio for the treatment of relapsed and refractory acute myeloid leukemia
- PMID: 21282541
- PMCID: PMC4520927
- DOI: 10.1200/JCO.2010.30.5961
First-in-man study of CPX-351: a liposomal carrier containing cytarabine and daunorubicin in a fixed 5:1 molar ratio for the treatment of relapsed and refractory acute myeloid leukemia
Abstract
Purpose: This phase I dose-escalation trial was performed to determine the maximum-tolerated dose, dose-limiting toxicities, and pharmacokinetics of CPX-351.
Patients and methods: CPX-351 induction was administered on days 1, 3, and 5 by 90-minute infusion to 48 relapsed or refractory patients with acute myeloid leukemia (AML) or high-risk myelodysplasia. Doses started at 3 units/m(2) with dose doublings in single-patient cohorts until a pharmacodynamic effect (treatment-related adverse events or reduction in bone marrow cellularity or blast count) was observed, followed by 33% escalations in three patient cohorts until dose-limiting toxicity (DLT) occurred.
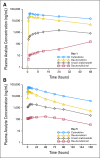
Results: The maximum-tolerated dose was 101 units/m(2). DLTs consisted of hypertensive crisis, congestive heart failure, and prolonged cytopenias. Adverse events were consistent with cytarabine and daunorubicin treatment. Response occurred at doses as low as 32 units/m(2). Of 43 patients with AML, nine had complete response (CR) and one had CR with incomplete platelet recovery; of patients with acute lymphoblastic leukemia, one of three had CR. Eight CRs were achieved among the 31 patients with prior cytarabine and daunorubicin treatment. CR in AML occurred in five of 26 patients age ≥ 60 years and in five of 17 patients younger than age 60 years. Median half-life was 31.1 hours (cytarabine) and 21.9 hours (daunorubicin), with both drugs and their metabolites detectable > 7 days after the last dose. The targeted 5:1 molar ratio was maintained at all dose levels for up to 24 hours.
Conclusion: The recommended dose of CPX-351 for phase II study is 101 units/m(2). Further exploration of efficacy and safety is ongoing in phase II trials in newly diagnosed and first-relapse patients with AML.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
References
-
- Schiffer CA, Stone RM. Acute myeloid leukemia in adults. In: Kufe DW, Bast RC, Hait WN, et al., editors. Cancer Medicine 7. Hamilton, Ontario, Canada: BC Decker; 2006. pp. 1739–1760.
-
- Löwenberg B, Ossenkoppele GJ, van Putten W, et al. High-dose daunorubicin in older patients with acute myeloid leukemia. N Engl J Med. 2009;361:1235–1248. - PubMed
-
- Goldie JH, Coldman AJ. Application of theoretical models to chemotherapy protocol design. Cancer Treat Rep. 1986;70:127–131. - PubMed
-
- Frei E, Eder JP. Principles of dose, schedule, and combination therapy. In: Kufe DW, Bast RC, Hait WN, et al., editors. Cancer Medicine 7. Hamilton, Ontario, Canada: BC Decker; 2006. pp. 590–599.
-
- Mayer LD, Harasym TO, Tardi PG, et al. Ratiometric dosing of anticancer drug combinations: Controlling drug ratios after systemic administration regulates therapeutic activity in tumor-bearing mice. Mol Cancer Ther. 5:1854–1863. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

