Enhancing patient-provider communication with the electronic self-report assessment for cancer: a randomized trial
- PMID: 21282548
- PMCID: PMC3068053
- DOI: 10.1200/JCO.2010.30.3909
Enhancing patient-provider communication with the electronic self-report assessment for cancer: a randomized trial
Abstract
Purpose: Although patient-reported cancer symptoms and quality-of-life issues (SQLIs) have been promoted as essential to a comprehensive assessment, efficient and efficacious methods have not been widely tested in clinical settings. The purpose of this trial was to determine the effect of the Electronic Self-Report Assessment-Cancer (ESRA-C) on the likelihood of SQLIs discussed between clinicians and patients with cancer in ambulatory clinic visits. Secondary objectives included comparison of visit duration between groups and usefulness of the ESRA-C as reported by clinicians.
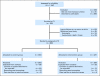
Patients and methods: This randomized controlled trial was conducted in 660 patients with various cancer diagnoses and stages at two institutions of a comprehensive cancer center. Patient-reported SQLIs were automatically displayed on a graphical summary and provided to the clinical team before an on-treatment visit (n = 327); in the control group, no summary was provided (n = 333). SQLIs were scored for level of severity or distress. One on-treatment clinic visit was audio recorded for each participant and then scored for discussion of each SQLI. We hypothesized that problematic SQLIs would be discussed more often when the intervention was delivered to the clinicians.
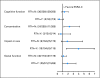
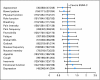
Results: The likelihood of SQLIs being discussed differed by randomized group and depended on whether an SQLI was first reported as problematic (P = .032). Clinic visits were similar with regard to duration between groups, and clinicians reported the summary as useful.
Conclusion: The ESRA-C is the first electronic self-report application to increase discussion of SQLIs in a US randomized clinical trial.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
Comment in
-
Supporting clinical practice decisions with real-time patient-reported outcomes.J Clin Oncol. 2011 Mar 10;29(8):954-6. doi: 10.1200/JCO.2010.33.2668. Epub 2011 Jan 31. J Clin Oncol. 2011. PMID: 21282536 No abstract available.
-
Perspectives of patients on the utility of electronic patient-reported outcomes on cancer care.J Clin Oncol. 2011 Nov 1;29(31):4213-4. doi: 10.1200/JCO.2011.37.9750. Epub 2011 Sep 19. J Clin Oncol. 2011. PMID: 21931027 No abstract available.
References
-
- US Food and Drug Administration. Guidance for industry: Patient-reported outcome measures— Use in medical product development to support labeling claims. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformati.... - PMC - PubMed
-
- Valderas JM, Kotzeva A, Espallargues M, et al. The impact of measuring patient-reported outcomes in clinical practice: A systematic review of the literature. Qual Life Res. 2008;17:179–193. - PubMed
-
- Detmar SB, Muller MJ, Schornagel JH, et al. Health related quality-of-life assessments and patient-physician communication: A randomized controlled trial. JAMA. 2002;288:3027–3034. - PubMed
-
- Bruera E. Routine symptom assessment: Good for practice and good for business. Support Care Cancer. 2008;16:537–538. - PubMed
-
- Carlson LE, Speca M, Hagen N, et al. Computerized quality-of-life screening in a cancer pain clinic. J Palliat Care. 2001;17:46–52. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

