Human papillomavirus testing in the prevention of cervical cancer
- PMID: 21282563
- PMCID: PMC3046952
- DOI: 10.1093/jnci/djq562
Human papillomavirus testing in the prevention of cervical cancer
Abstract
Strong evidence now supports the adoption of cervical cancer prevention strategies that explicitly focus on persistent infection with the causal agent, human papillomavirus (HPV). To inform an evidence-based transition to a new public health approach for cervical cancer screening, we summarize the natural history and cervical carcinogenicity of HPV and discuss the promise and uncertainties of currently available screening methods. New HPV infections acquired at any age are virtually always benign, but persistent infections with one of approximately 12 carcinogenic HPV types explain virtually all cases of cervical cancer. In the absence of an overtly persistent HPV infection, the risk of cervical cancer is extremely low. Thus, HPV test results predict the risk of cervical cancer and its precursors (cervical intraepithelial neoplasia grade 3) better and longer than cytological or colposcopic abnormalities, which are signs of HPV infection. The logical and inevitable move to HPV-based cervical cancer prevention strategies will require longer screening intervals that will disrupt current gynecologic and cytology laboratory practices built on frequent screening. A major challenge will be implementing programs that do not overtreat HPV-positive women who do not have obvious long-term persistence of HPV or treatable lesions at the time of initial evaluation. The greatest potential for reduction in cervical cancer rates from HPV screening is in low-resource regions that can implement infrequent rounds of low-cost HPV testing and treatment.
Figures

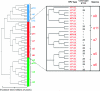




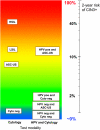
Comment in
-
Re: Human papillomavirus testing in the prevention of cervical cancer.J Natl Cancer Inst. 2011 Oct 5;103(19):1482-3; author reply 1483-4. doi: 10.1093/jnci/djr308. Epub 2011 Aug 22. J Natl Cancer Inst. 2011. PMID: 21859986 No abstract available.
References
-
- Gustafsson L, Pontén J, Bergström R, Adami HO. International incidence rates of invasive cervical cancer before cytological screening. Int J Cancer. 1997;71(2):159–165. - PubMed
-
- Gustafsson L, Pontén J, Zack M, Adami HO. International incidence rates of invasive cervical cancer after introduction of cytological screening. Cancer Causes Control. 1997;8(5):755–763. - PubMed
-
- Saslow D, Runowicz CD, Solomon D, et al. American Cancer Society guideline for the early detection of cervical neoplasia and cancer. CA Cancer J Clin. 2002;52(6):342–362. - PubMed
-
- Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet. 2007;370(9590):890–907. - PubMed
-
- International Agency for Research on Cancer. Cervix Cancer Screening. Lyon, France: IARC Press; 2005.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

