Losartan inhibits collagen I synthesis and improves the distribution and efficacy of nanotherapeutics in tumors
- PMID: 21282607
- PMCID: PMC3041115
- DOI: 10.1073/pnas.1018892108
Losartan inhibits collagen I synthesis and improves the distribution and efficacy of nanotherapeutics in tumors
Abstract
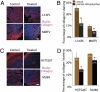
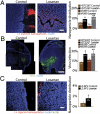
The dense collagen network in tumors significantly reduces the penetration and efficacy of nanotherapeutics. We tested whether losartan--a clinically approved angiotensin II receptor antagonist with noted antifibrotic activity--can enhance the penetration and efficacy of nanomedicine. We found that losartan inhibited collagen I production by carcinoma-associated fibroblasts isolated from breast cancer biopsies. Additionally, it led to a dose-dependent reduction in stromal collagen in desmoplastic models of human breast, pancreatic, and skin tumors in mice. Furthermore, losartan improved the distribution and therapeutic efficacy of intratumorally injected oncolytic herpes simplex viruses. Finally, it also enhanced the efficacy of i.v. injected pegylated liposomal doxorubicin (Doxil). Thus, losartan has the potential to enhance the efficacy of nanotherapeutics in patients with desmoplastic tumors.
Conflict of interest statement
Conflict of interest statement: R.K.J. received commercial research grants from Dyax, AstraZeneca, and MedImmune; consultant fees from AstraZeneca/MedImmune, Dyax, Astellas-Fibrogen, Regeneron, Genzyme, Morphosys, and Noxxon Pharma; and a speaker honorarium from Genzyme. R.K.J. owns stock in SynDevRx. No reagents or funding from these companies was used in these studies. There is no significant financial or other competing interest in the work.
Figures
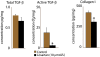




References
-
- Davis ME, Chen ZG, Shin DM. Nanoparticle therapeutics: An emerging treatment modality for cancer. Nat Rev Drug Discov. 2008;7:771–782. - PubMed
-
- Peer D, et al. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2:751–760. - PubMed
-
- Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4:145–160. - PubMed
-
- Netti PA, Berk DA, Swartz MA, Grodzinsky AJ, Jain RK. Role of extracellular matrix assembly in interstitial transport in solid tumors. Cancer Res. 2000;60:2497–2503. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

