XMAP215 polymerase activity is built by combining multiple tubulin-binding TOG domains and a basic lattice-binding region
- PMID: 21282620
- PMCID: PMC3041093
- DOI: 10.1073/pnas.1016498108
XMAP215 polymerase activity is built by combining multiple tubulin-binding TOG domains and a basic lattice-binding region
Abstract
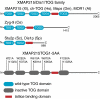
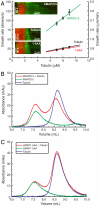
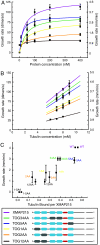
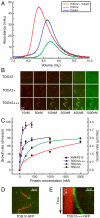
XMAP215/Dis1 family proteins positively regulate microtubule growth. Repeats at their N termini, called TOG domains, are important for this function. While TOG domains directly bind tubulin dimers, it is unclear how this interaction translates to polymerase activity. Understanding the functional roles of TOG domains is further complicated by the fact that the number of these domains present in the proteins of different species varies. Here, we take advantage of a recent crystal structure of the third TOG domain from Caenorhabditis elegans, Zyg9, and mutate key residues in each TOG domain of XMAP215 that are predicted to be important for interaction with the tubulin heterodimer. We determined the contributions of the individual TOG domains to microtubule growth. We show that the TOG domains are absolutely required to bind free tubulin and that the domains differentially contribute to XMAP215's overall affinity for free tubulin. The mutants' overall affinity for free tubulin correlates well with polymerase activity. Furthermore, we demonstrate that an additional basic region is important for targeting to the microtubule lattice and is critical for XMAP215 to function at physiological concentrations. Using this information, we have engineered a "bonsai" protein, with two TOG domains and a basic region, that has almost full polymerase activity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Howard J, Hyman AA. Microtubule polymerases and depolymerases. Curr Opin Cell Biol. 2007;19:31–35. - PubMed
-
- Pantaloni D, Le Clainche C, Carlier MF. Mechanism of actin-based motility. Science. 2001;292:1502–1506. - PubMed
-
- Desai A, Mitchison TJ. Microtubule polymerization dynamics. Annu Rev Cell Dev Biol. 1997;13:83–117. - PubMed
-
- Goode BL, Drubin DG, Barnes G. Functional cooperation between the microtubule and actin cytoskeletons. Curr Opin Cell Biol. 2000;12:63–71. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

